Vietnam Congestive Heart Failure Therapeutics Market Analysis
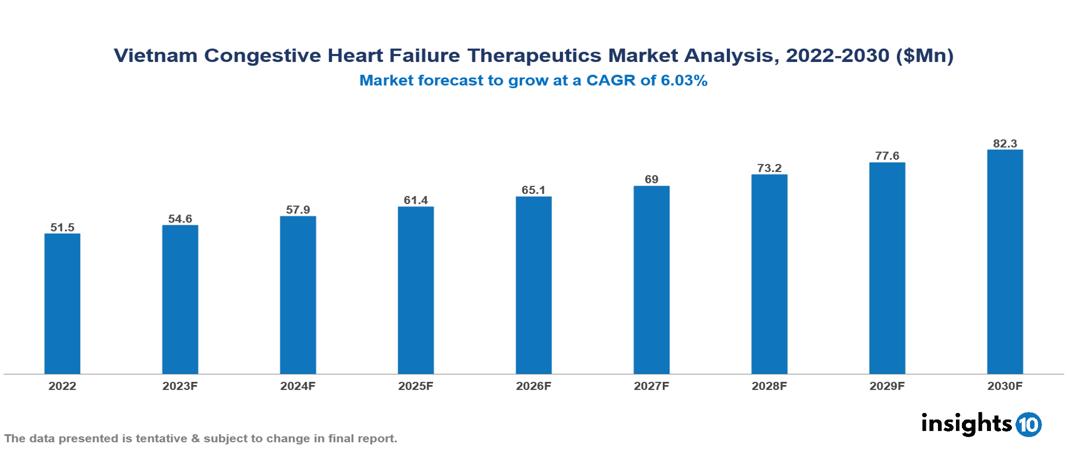
The Vietnam Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $51.5 Mn in 2022 to $82.3 Mn by 2030, with a CAGR of 6.03% during the forecast period of 2022-2030. The drivers include the increase in CHF prevalence due to aging populations, unhealthy lifestyles, and improved diagnostics, coupled with heightened awareness, early detection efforts, and persistent unmet medical needs among CHF patients, creating opportunities for innovative therapeutics. The Vietnam Congestive Heart Failure Therapeutics Market encompasses various key players across different therapeutic segments, including AstraZeneca, Novartis, Sanofi, Abbott Laboratories, Pfizer, Traphaco, Mekophar, Domesco, Hau Giang Pharmaceutical, Pharmedic, etc., among various others.
Buy Now

Vietnam Congestive Heart Failure Therapeutics Market Analysis Executive Summary
The Vietnam Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $51.5 Mn in 2022 to $82.3 Mn by 2030, with a CAGR of 6.03% during the forecast period of 2022-2030.
Chronic Heart Failure (CHF), also known as heart failure, is a medical condition where the heart's ability to pump blood is compromised, leading to inadequate circulation and oxygen delivery to the body's tissues. Common causes include heart attacks, hypertension, and various cardiac diseases. Symptoms include shortness of breath, fatigue, and fluid retention. Treatment for CHF aims to manage symptoms, improve the heart's function, and enhance patients' overall quality of life. Medications play a key role, including diuretics to reduce fluid retention, angiotensin-converting enzyme (ACE) inhibitors to relax blood vessels, and beta-blockers to lower heart rate and blood pressure. Additionally, aldosterone antagonists and inotropic agents may be prescribed to address specific aspects of heart failure. Lifestyle modifications such as a heart-healthy diet, regular exercise, and sodium restriction are crucial components of CHF management. In advanced cases, surgical interventions like heart valve repair, coronary artery bypass grafting, or even heart transplant may be considered. The comprehensive approach to CHF treatment emphasizes a combination of medications, lifestyle adjustments, and, in severe cases, surgical interventions to optimize patient outcomes and slow the progression of this chronic cardiovascular condition.
In Vietnam, cardiovascular diseases account for 20% to 30% of all fatalities. Approximately 18.9% of adults between the ages of 18 and 69 have hypertension, the primary risk factor for congestive heart failure. The main contributors to CVDs are bad eating habits, hazardous alcohol consumption, physical inactivity, and tobacco use.
The drivers include the increase in CHF prevalence due to aging populations, unhealthy lifestyles, and improved diagnostics, coupled with heightened awareness, early detection efforts, and persistent unmet medical needs among CHF patients, creating opportunities for innovative therapeutics.
One of the biggest pharmaceutical businesses in Vietnam, Traphaco is well-known in the cardiovascular market. They produce a large variety of generic CHF drugs, such as beta-blockers, ARBs, diuretics, and ACE inhibitors. Mekophar: Another well-known pharmaceutical firm in Vietnam, with an expanding line of cardiovascular goods. Global brands AstraZeneca, Novartis, and Sanofi have developed marketing networks and enhanced research and development in novel pharmaceuticals.
Market Dynamics
Market Growth Drivers
Escalating CHF Incidence: The global surge in CHF prevalence is an evident trend attributed to aging populations, unhealthy lifestyle choices, and enhanced diagnostic capabilities. Vietnam mirrors this pattern, with the burgeoning number of CHF cases underscoring the potential escalation in demand for CHF therapeutics.
Increased Awareness and Early Detection: The heightened awareness surrounding CHF, coupled with advancements in diagnostic methodologies, is poised to facilitate earlier detection and treatment initiation. This positive shift could not only improve patient outcomes but also contribute to the expansion of the market for CHF drugs, as proactive healthcare measures gain prominence.
Persistent Unmet Medical Needs: Despite the availability of current treatment modalities, a substantial unmet medical need persists among CHF patients. This gap in addressing the diverse needs of individuals with CHF creates a fertile ground for the development of novel and innovative therapeutics. Opportunities abound for research and development efforts to introduce interventions that effectively meet the unique challenges and complexities faced by individuals grappling with CHF.
Market Restraints
Elevated Pharmaceutical Expenses: The exorbitant prices associated with branded CHF drugs pose a significant impediment to accessibility, especially in developing nations such as Vietnam. The substantial financial burden imposed by these high drug costs can hinder patients' ability to obtain essential medications and compromise their overall health outcomes.
Constrained Healthcare Budgets: The restricted financial resources allocated to government healthcare budgets in Vietnam present a challenge in prioritizing and affording expensive CHF medications. This limitation may lead to difficulties in ensuring widespread access to vital treatments, potentially impacting the overall management of CHF within the healthcare system.
Proliferation of Counterfeit Drugs: The prevalence of counterfeit drugs in the pharmaceutical market raises concerns about the authenticity and safety of medications. In the context of CHF, the presence of counterfeit drugs not only undermines the trust in legitimate pharmaceuticals but also poses serious safety risks to patients. Addressing this issue is crucial for safeguarding public health and ensuring that individuals can trust the medications prescribed for managing CHF.
Healthcare Policies and Regulatory Landscape
In Vietnam, the healthcare sector is significantly influenced by pivotal healthcare policies aimed at enhancing accessibility and quality of services for the population. The government takes an active role in funding the healthcare system, with policies addressing various aspects such as infrastructure development, healthcare workforce training, and disease prevention initiatives. Oversight of drug registration, quality control, and post-marketing surveillance is entrusted to the Drug Administration of Vietnam (DAV), a crucial regulatory body ensuring public health by enforcing pharmaceutical industry regulations. The DAV collaborates with international organizations, adhering to global standards to strengthen the regulatory framework and promote a resilient pharmaceutical sector. Their vigilance is crucial in guaranteeing that only safe and effective medications reach the market, contributing significantly to the well-being of the Vietnamese people.
Competitive Landscape
Key Players:
- AstraZeneca
- Novartis
- Sanofi
- Abbott Laboratories
- Pfizer
- Traphaco
- Mekophar
- Domesco
- Hau Giang Pharmaceutical
- Pharmedic
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Vietnam Congestive Heart Failure Therapeutics Market Segmentation
By Stage of Heart Failure
- Acute Heart Failure
- Chronic Heart Failure
By Drug Class
- ACE Inhibitors
- Beta Blockers
- Angiotensin 2 Receptor Blockers
- Diuretics
- Aldosterone Antagonists
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.