Vietnam Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Analysis
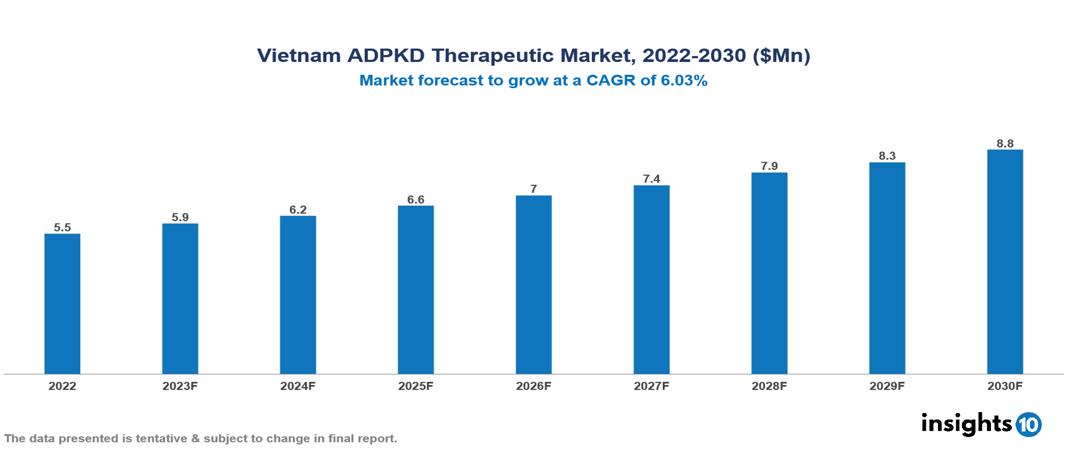
The Vietnam Autosomal Dominant Polycystic Kidney Disease Therapeutics Market was valued at US $6 Mn in 2022 and is predicted to grow at a CAGR of 6.03% from 2023 to 2030, to US $9 Mn by 2030. The key drivers of this industry include the upward trend in the prevalence of autosomal dominant polycystic kidney disease, government initiatives, increased awareness, and other factors. The industry is primarily dominated by players such as Otsuka, AceLink, Palladio, Reata, Xortx, Regulus, Exelixis, Sanofi and other players
Buy Now

Vietnam Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Analysis
The Vietnam Autosomal Dominant Polycystic Kidney Disease Therapeutics Market is at around US $6 Mn in 2022 and is projected to reach US $9 Mn in 2030, exhibiting a CAGR of 6.03% during the forecast period.
Mutations in the PKD1 and PKD2 genes produce autosomal dominant polycystic kidney disease (ADPKD), a hereditary disorder affecting several organs. This causes the kidneys to enlarge(renomegaly) as fluid-filled cysts begin to form in them. The majority of ADPKD cases result in renal failure (ESKD). Fatigue, discomfort, and recurrent infections are typical symptoms. This rare condition has no known cure, although symptoms can be managed and complications can be avoided with treatment. The only medication approved for ADPKD is tolvaptan (Jinarc), made by Otsuka Pharmaceuticals, which is effective in certain patients by acting as a vasopressin blocker to decrease the formation of cysts. Pain management and lifestyle changes are additional therapy possibilities. A number of treatments have been developed to treat ADPKD, such as mTOR inhibitors (Everolimus, Sirolimus), however, they have not been successful in preventing the disease progression.
The evidence suggests the overall prevalence of ADPKD is around 1 in 400 to 1 in 1000 individuals in Vietnam. It is anticipated that the number of ADPKD patients will rise due to factors including longer life expectancies and an aging population, as well as modifiable risk factors like dietary changes, lifestyle adjustments, and physical inactivity. The market is therefore driven by major factors like rising prevalence, a shifting healthcare landscape, improved access, and evolving treatment options in the industry. However, conditions such as high costs of treatment, a lack of specialists like nephrologists, and others hinder the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Surge in the prevalence of ADPKD: According to several estimates, the expected prevalence of ADPKD is around 1 in 400 to 1 in 1000 individuals in Vietnam. Factors such as increased life span and geriatric population, along with modifiable risk factors like diet, lifestyle changes, and physical inactivity, are expected to increase the patient pool of ADPKD patients requiring advanced treatments, resulting in the growth of the market.
Shifting healthcare landscape: Vietnam's healthcare system is evolving, providing improved access to specialized care and diagnostic resources, potentially energizing the ADPKD therapeutics market. As the economy grows, healthcare spending in Vietnam is expected to increase, enhancing the accessibility of ADPKD treatments and broadening their market presence.
Evolving treatment options: The development of novel therapies aimed at reducing ADPKD progression and combined treatments targeting different aspects of the condition could establish untapped market segments. Increased awareness among healthcare providers and patients regarding ADPKD and the available treatments drives market growth.
Increased awareness: Increasing public awareness and educational campaigns promote early diagnosis, enabling timely interventions and potentially widening the market for treatments focused on slowing the advancement of the disease. Groups such as the Vietnam Kidney Foundation and the Vietnam Association of Rare Diseases work towards improving patient access and care, influencing evidence-based policy decisions by gathering data that propels the therapeutics market.
Market Restraints
High costs of treatment: Innovative treatments for ADPKD, notably gene therapy approaches, may incur significant costs. Despite insurance coverage by the government, patients might encounter financial challenges. Currently, there's a limited implementation of tiered pricing systems that offer subsidized rates for essential medications, specifically ADPKD drugs, in Vietnam. This situation could restrict treatment access and hinder market advancement.
Lack of specialists: In rural areas of Vietnam, patients with ADPKD face restricted access to specialized care and suitable treatment options due to the concentration of nephrologists and other experts in major cities. The disproportionate number of patients compared to available specialists can curtail consultation time and the quality of care provided to individuals with ADPKD.
Socioeconomic conditions: Financial hardship, absence of social support, and restricted educational opportunities adversely affect the capacity of ADPKD patients to handle their condition and result in non-compliance with treatment regimens that hamper market growth.
Healthcare Policies and Regulatory Landscape
Vietnam's healthcare policy and regulatory system encompass multiple crucial entities and bodies. The principal authority overseeing healthcare regulations and licensing in Vietnam is the Drug Administration of Vietnam, operating under the Ministry of Health (MOH). The MOH bears the responsibility of formulating national health policies, orchestrating healthcare reforms, and supervising healthcare services.
Acquiring a license for healthcare products in Vietnam necessitates compliance with the MOH's regulations. Companies dealing with pharmaceuticals and medical devices must obtain registration and marketing approval from the MOH. This process involves presenting technical and scientific data to validate the safety, quality, and effectiveness of the product.
Vietnam's healthcare policy and regulatory framework involve various agencies and bodies, with the MOH playing a central role in regulating healthcare products. Both the public and private healthcare sectors in the country present diverse opportunities for companies engaged in the healthcare industry.
Competitive Landscape
Key Players
- Otsuka Pharmaceutical Co., Ltd
- Regulus Therapeutics
- Palladio Biosciences
- Reata Pharmaceuticals
- Galapagos NV
- Exelixis Inc
- AceLink Therapeutics, Inc
- Sanofi
- Xortx Therapeutics
- Pano Therapeutics
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Vietnam Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Segmentation
By Treatment
- Pain & Inflammation Treatment
- Kidney Stone Treatment
- Urinary Tract Infection Treatment
- Kidney Failure Treatment
By Route of Administration
- Oral
- Parenteral
- Others
By End User
- Hospitals
- Speciality Clinics
- Surgical Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.