US Genomic Diagnostics Market Analysis
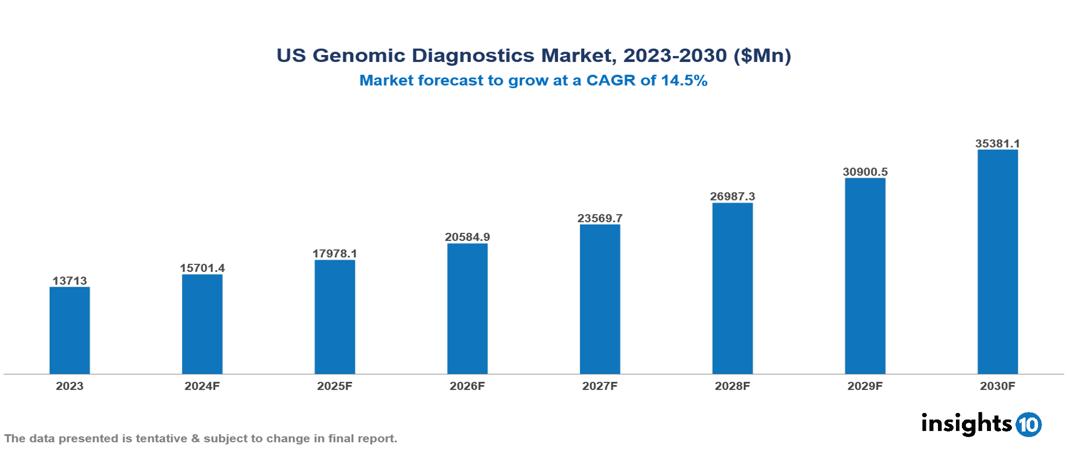
US Genomic Diagnostics Market was valued at $13,713 Mn in 2023 and is predicted to grow at a CAGR of 14.5% from 2023 to 2030, to $35,381.06 Mn by 2030. The key drivers of this industry include strong demand for early & preventive care, precision medicine & personalized treatments, and government funding & initiatives. The industry is primarily dominated by Illumina, 23andMe, Myriad Genetics, and Amgen among others.
Buy Now

US Genomic Diagnostics Market Executive Summary
US Genomic Diagnostics Market was valued at $13,713 Mn in 2023 and is predicted to grow at a CAGR of 14.5% from 2023 to 2030, to $35,381.06 Mn by 2030.
Genomic diagnostics is a rapidly evolving field that uses an individual's genetic information to diagnose diseases, assess predisposition to future health problems, and guide treatment plans by analyzing DNA or RNA for disease-linked variations. This includes karyotyping to examine chromosome abnormalities, targeted mutation analysis for specific disease-related genes, and next-generation sequencing (NGS) for a comprehensive genetic analysis. Applications encompass disease diagnosis, carrier testing for informed family planning, predictive testing for disease risk assessment, and pharmacogenomics for personalized medication treatments. The benefits of genomic diagnostics include early disease detection, personalized medicine, and improved disease management and prognosis.
In the US, approximately 129 Mn people live with at least one major chronic disease in 2022, with 42% of adults having two or more chronic conditions and 12% having five or more. Chronic diseases account for about 70% of all deaths, equating to over 1.7 Mn deaths annually, with heart disease and cancer responsible for nearly 40% of these fatalities. The prevalence of chronic conditions has increased alongside the aging population, with the average age rising from 29.5 years in 1960 to 38.6 years in 2021. Genomic diagnostics are emerging as vital tools in chronic disease management, offering insights into genetic predispositions and enabling personalized treatment and early intervention, thus improving outcomes and preventive care.
Market is therefore driven by significant factors like strong demand for early & preventive care, precision medicine & personalized treatments, and government funding & initiatives. However, data privacy concerns, reimbursement challenges, and limited expertise & infrastructure restrict the growth and potential of the market.
A prominent player in this field is Illumina, which has partnered with AstraZeneca to leverage genomics and AI for faster drug development by identifying new therapeutic targets and biomarkers, 23andMe acquired Lemonaid Health to enhance its personalized healthcare offerings through telehealth and prescription drug delivery services based on genetic information. Other contributors include Myriad Genetics, and Amgen among others.
Market Dynamics
Market Growth Drivers
Strong Demand for Early & Preventive Care: Rising healthcare costs and the chronic disease burden emphasize early detection. Genomic testing, which helps identify risks early and enables preventive measures, is crucial. The CDC estimates chronic diseases cost $320 Bn annually.
Precision Medicine & Personalized Treatments: Genomics allows for the tailoring of treatments to an individual's genetic makeup. A 2022 study highlights the cost-effectiveness of pharmacogenomics testing, making personalized medicine a significant market driver.
Government Funding & Initiatives: The US government invests heavily in genomics research, with the National Human Genome Research Institute (NHGRI) budget exceeding $600Mn in 2023. This funding fosters innovation and broader adoption of genomic technologies.
Market Restraints
Data Privacy Concerns: Public concerns about the control and security of genetic data are significant. A 2023 Pew Research survey found that 72% of Americans are worried about unauthorized access to their genetic information, making addressing these concerns crucial.
Reimbursement Challenges: Insurance coverage for genomic tests can be complex and varies depending on the test and its clinical justification. A 2022 report by the American College of Medical Genetics and Genomics highlights the challenges in reimbursement policies, which limit patient access.
Limited Expertise & Infrastructure: The field requires specialized personnel for interpreting tests, and a shortage of genetic counselors and lab staff can create bottlenecks. Investments in workforce development are needed to overcome these limitations.
Regulatory Landscape and Reimbursement Scenario
In the US genomic diagnostics market, several key regulatory bodies play crucial roles. The Food and Drug Administration (FDA) oversees the safety and efficacy of genomic tests, categorizing them based on risk and requiring varying levels of premarket review. The Centers for Medicare & Medicaid Services (CMS) regulate laboratories through the Clinical Laboratory Improvement Amendments (CLIA) program, ensuring quality and accuracy in testing. Additionally, the Federal Trade Commission (FTC) monitors the marketing of genetic tests to prevent misleading claims.
Reimbursement for genomic tests involves multiple stakeholders and is characterized by complexity. Private insurance companies decide coverage based on medical necessity and clinical utility, while Medicare and Medicaid have specific rules that often require evidence of clinical benefit for coverage. Patients may face out-of-pocket costs if their insurance does not fully cover the tests. Challenges in reimbursement include the rapid pace of technological advancement, lack of standardized guidelines for medical necessity, and the high cost of some tests, which can limit patient access even with insurance.
Competitive Landscape
Key Players
Here are some of the major key players in the US Genomic Diagnostics
- Illumina, Inc.
- Myriad Genetics, Inc.
- Amgen, Inc.
- 23andMe
- AncestryDNA
- Helix OpCo LLC
- Color Genomics, Inc.
- Everly Well
- MyDNA
- Igenomix
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Genomic Diagnostics Market Segmentation
By Technology
- Next Generation Sequencing
- Array Technology
- PCR-based Testing
- FISH
- Others
By Application
- Ancestry & Ethnicity
- Traits Screening
- Genetic Disease Carrier Status
- New Baby Screening
- Health and Wellness-Predisposition/Risk/Tendency
By Product
- Consumables
- Equipment
- Software & Services
By End-user
- Hospitals & Clinics
- Diagnostic Laboratories
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































