US Financial Assistance Programs Market Analysis
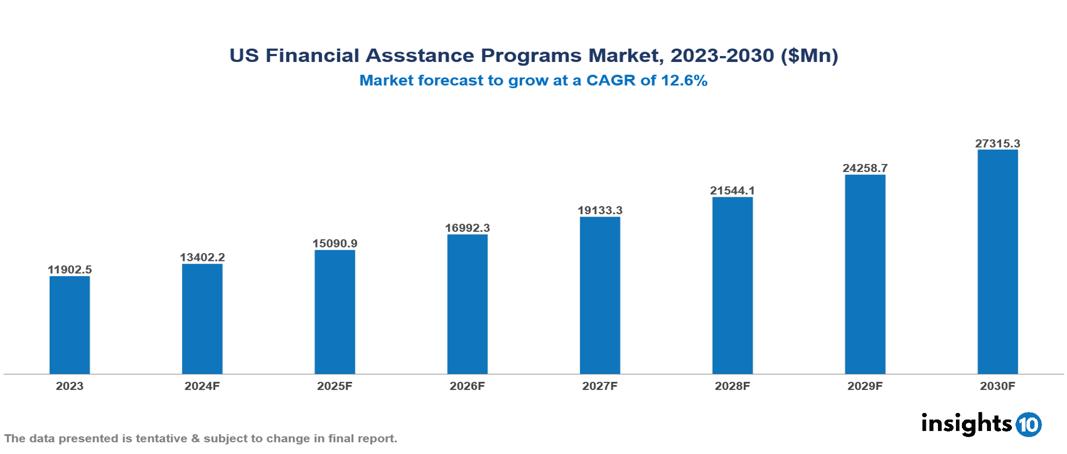
The US Financial Assistance Programs Market was valued at $11,902.5 Mn in 2023 and is projected to grow at a CAGR of 12.6% from 2023 to 2023, to $27,315.6 Mn by 2030. The market is driven by various sector such as rising drug cost, complex insurance landscape, regulatory environment, market competition, patient adherence concern etc. The prominent pharmaceutical companies providing financial assistance to patient are such as Merck, GSK, Johnson & Johnson, Novartis among others.
Buy Now

US Financial Assistance Programs Market Executive Summary
The US Financial Assistance Programs Market is at around $11,902.5 Mn in 2023 and is projected to reach $27,315.6 Mn in 2030, exhibiting a CAGR of 12.6% during the forecast period 2023-2030.
The aim of drug manufacturers' patient financial support is to minimize or remove out-of-pocket cost sharing as an obstacle when patients choose medications, thereby keeping them on brand-name drugs for longer. Co-pay assistance, free drugs trails, bridge programs, sliding scale programme, Coupons, Bulk purchasing programs etc are some of the widely used financial assistance programme under patient assistance programs. Patients' out-of-pocket drug cost sharing is determined by their health plans or pharmacy benefit manager's (PBM's) formulary--a list of preferred and nonpreferred prescription drugs. Preferred status is based on a drug's effectiveness, price, and the level of rebate the payer receives from the manufacturer for giving the drug preference over its competitors. Generics and preferred brand drugs are generally assigned lower patient cost sharing than nonpreferred brand drugs. As drug prices have increased, so has patient cost sharing, causing some patients to stretch, forgo, or discontinue medication that is too expensive. Drug manufacturers often seek to mitigate these effects by providing or funding various forms of patient financial support.
According to Centre for disease control and prevention, an estimated 129 Mn people in the US have at least 1 major chronic disease that is heart disease, cancer, diabetes, obesity, hypertension as defined by the US Department of Health and Human Services. Therefore, the market is predominately driven by factors such as increasing prevalence of chronic conditions, technological advancements, and increasing patient awareness where factors such as complex insurance landscape, stringent regulatory environment and budgetary concerns restrict the market
Pharmaceutical companies providing financial assistance to patient are such as Merck, GSK, Pfizer, Johnson & Johnson, Novartis among others.
Market Dynamics
Market Drivers
Patient Advocacy and Awareness: Pharmaceutical companies are recognizing the importance of patient advocacy and awareness in their financial assistance programs. By educating patients about their conditions and treatment options, pharmaceutical companies can help patients better manage their health, leading to improved outcomes and reduced healthcare costs.
Technological Advancements: The adoption of advanced technologies like AI, machine learning, and sensors in pharmaceutical manufacturing and research can lead to improved efficiency and reduced costs, making financial assistance more feasible. This can help pharmaceutical companies develop innovative medicines and treatments, which are essential for managing chronic diseases.
Chronic disease prevalence: According to Centre for disease control and prevention, an estimated 129 Mn people in the US have at least 1 major chronic disease that is heart disease, cancer, diabetes, obesity, hypertension as defined by the US Department of Health and Human Services. This increasing number of patients with chronic conditions necessitates long-term, often expensive treatments. This creates a sustained need for financial assistance over extended periods. Chronic disease management is a priority in healthcare, driving support for assistance programs.
Market Restraints
Insurance pushback: Some insurers argue that assistance programs interfere with their cost-management strategies and formulary structures. There is a growing trend where insurers are excluding copay assistance from contributing to patients' deductibles through copay accumulator programs. This resistance may reduce the impact and accessibility of assistance programs for certain patients, potentially limiting their benefits.
Budgetary pressures: Pharmaceutical companies face the challenge of balancing the costs of assistance programs while safeguarding profit margins. Economic downturns or shifts in company strategy can lead to a reduction in funding for these programs. As a result, companies may become more selective, limiting the scope of their assistance offerings.
Regulatory Landscape and Reimbursement Scenario
The Centres for Disease Control and Prevention (CDC) serves as the primary regulatory body in the United States that oversees pharmaceutical products and diagnostic tools, while also playing a vital role in raising awareness about the disease among the general public. Although the Food and Drug Administration (FDA) is responsible for approving drugs and diagnostic tests, the CDC provides guidance and recommendations on their appropriate use for chronic disease prevention and treatment.
The CDC closely monitors the incidence and prevalence of chronic diseases cases in the U.S. through its surveillance systems, and this data guides public health policies, resource allocation, and awareness efforts. Additionally, the CDC develops and promotes evidence-based strategies for preventing the spread of chronic diseases, including vaccination programs, safe injection practices, and harm reduction strategies.
Furthermore, the CDC plays a crucial role in raising public awareness through educational campaigns, community outreach programs, and dissemination of informational materials, aiming to increase knowledge about transmission, symptoms, testing, and available resources. Importantly, the CDC collaborates with state and local health departments, community organizations, and other stakeholders to coordinate prevention, control, and awareness efforts across the nation.
Competitive Landscape
Key Players
Here are some of the major key players in the US Financial Assistance Programs Market:
- Roche
- Pfizer
- Bayer
- Sanofi
- Novartis
- Merck
- GSK
- Johnson & Johnson
- AbbVie
- AstraZeneca
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Financial Assistance Programs Market Segmentation
By Application
- Population Health Management
- Outpatient Health Management
- In-patient Health Management
- Others
By Therapeutics Area
- Health & Wellness
- Chronic Disease Management
- Other therapeutic area
By End Users
- Payers
- Providers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































