US Diabetes Drugs Market Analysis
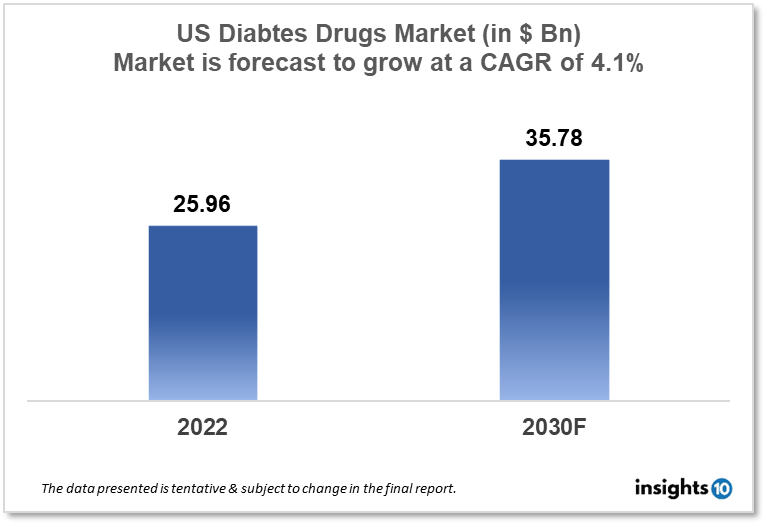
The US diabetes drugs market size was valued at $25.94 Bn in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 4.1% from 2022 to 2030 and will reach $35.78 Bn in 2030. The market is segmented by drug type, application, and distribution channel. The US diabetes drug market will grow because the prevalence of diabetes in the US is a major driver of the market. There is a large and growing demand for diabetes medications, including insulin and oral medications, to manage the disease and prevent its complications. The key market players are Johnson & Johnson, Novo Nordisk, Novartis, Merck, Eli Lilly and Company, and others.
Buy Now

US Diabetes Drugs Market Executive Summary
The US diabetes drugs market size was valued at $25.94 Bn in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 4.1% from 2022 to 2030 and will reach $35.78 Bn in 2030. The United States has one of the highest health expenditures in the world, both in absolute terms and as a percentage of its gross domestic product (GDP). According to data from the Centers for Medicare and Medicaid Services (CMS), US healthcare spending totaled $3.8 trillion in 2019 or $11,582 per person. This represented approximately 17.7% of the US GDP.
There are several factors that contribute to high health expenditures in the US. One of the biggest drivers is the high cost of medical services and treatments. The US has some of the highest healthcare prices in the world, including prescription drugs, medical devices, and hospital care. Additionally, the US has a higher prevalence of chronic diseases, such as diabetes, heart disease, and obesity, which require ongoing medical treatment and management.
Diabetes is a significant public health issue in the United States, affecting Mns of people and placing a significant burden on the healthcare system. According to the Centers for Disease Control and Prevention (CDC), in 2020, approximately 34.2 Mn people in the US, or 10.5% of the population, had diabetes. This includes both diagnosed and undiagnosed cases. The incidence of diabetes, or the rate at which new cases of the disease are diagnosed, has also been increasing in recent years. According to the CDC, in 2018, there were approximately 1.5 Mn new cases of diabetes diagnosed in the US.
The high prevalence and incidence of diabetes in the US has a significant impact on the diabetes drugs market. There is a large and growing demand for diabetes medications, including insulin and oral medications, to manage the disease and prevent its complications. In 2019, the global diabetes drugs market was valued at $65.4 Bn, and the US was the largest market for diabetes drugs, accounting for a significant share of the market.
The demand for diabetes medications has led to a highly competitive and innovative diabetes drug market in the US. There are numerous pharmaceutical companies that produce diabetes drugs, ranging from large multinational corporations to smaller specialty companies. The market includes both branded and generic drugs, and there is a significant investment in research and development to develop new and more effective treatments for diabetes.
Market Dynamics
Market Growth Drivers
- High prevalence of diabetes: The prevalence of diabetes in the US is a major driver of the market. There is a large and growing demand for diabetes medications, including insulin and oral medications, to manage the disease and prevent its complications.
- Ongoing need for treatment and management: Diabetes is a chronic disease that requires ongoing treatment and management, which creates a sustained demand for diabetes medications.
- Advances in diabetes drug research and development: There is a significant investment in research and development to develop new and more effective treatments for diabetes, which has led to the development of new classes of diabetes medications.
- Increasing awareness and education: Increased awareness and education about diabetes and its complications has led to greater demand for diabetes medications and improved treatment and management of the disease.
Market Restraints
- High cost of diabetes medications: The high cost of diabetes medications, including insulin, is a significant barrier to access and affordability for many people with diabetes.
- Regulatory issues: The approval process for new diabetes medications can be lengthy and expensive, which can slow down innovation and limit competition in the market.
- Competition from alternative therapies: Alternative therapies, such as lifestyle modifications and non-pharmacological interventions, can also be effective in managing diabetes, which can limit demand for diabetes medications.
- Patent expirations: The expiration of patents for some diabetes medications can result in increased competition from generic drugs, which can reduce revenue for pharmaceutical companies.
Competitive Landscape
Key Players
- Johnson & Johnson
- Bayer Pharmaceuticals
- Novo Nordisk
- Sanofi
- Merck
- Eli Lilly and Company
- AstraZeneca
- Takeda Pharmaceutical Company Limited
- Biocon ltd
- Bristol-Myers Squibb
- Boehringer Ingelheim
- Novartis
- GlaxoSmithKline
- Lupin ltd
- Piramal healthcare ltd
- Ranbaxy laboratories ltd.
Recent Developments
In 2021, the US FDA approved several new drugs for the treatment of diabetes. These include Zegalogue, a new formulation of glucagon for the treatment of severe hypoglycemia, and Wegovy, a once-weekly injection for the treatment of type 2 diabetes and obesity.
Healthcare Policies and Regulatory Landscape
Policy changes and Reimbursement scenario
The development and approval of diabetes drugs in the United States is regulated by the US Food and Drug Administration (FDA). The FDA is responsible for ensuring that drugs are safe and effective for their intended use, and that they meet certain standards of quality.
Before a new diabetes drug can be approved for use in the US, it must go through a rigorous testing and approval process. This process typically involves three phases of clinical trials, in which the drug is tested for safety and efficacy in human subjects. The FDA reviews the results of these clinical trials and other data to determine whether to approve the drug for use. Once a diabetes drug is approved by the FDA, it can be marketed and sold in the US. However, the FDA continues to monitor the drug's safety and efficacy after it is approved, and can take action to withdraw or restrict the drug if new safety concerns arise.
In addition to the FDA's regulatory oversight, there are also regulations governing the marketing and promotion of diabetes drugs in the US. Pharmaceutical companies must follow certain guidelines and restrictions when advertising their products to healthcare providers and consumers. There are also regulations governing the import and export of diabetes drugs in the US, which are designed to ensure that drugs are safe and of high quality. These regulations include requirements for labeling and packaging, as well as for testing and inspection of imported drugs.
In the US, reimbursement for diabetes drugs is primarily provided through health insurance plans, including private insurance, Medicare, and Medicaid. The reimbursement process can be complex, and can depend on factors such as the patient's insurance coverage, the specific diabetes drug being prescribed, and the prescribing physician's practice.
One of the key factors that affects reimbursement for diabetes drugs is the patient's insurance coverage. Many health insurance plans require patients to pay a portion of the cost of their medications, either through co-payments or deductibles. The amount of the patient's out-of-pocket expenses can vary depending on the specific insurance plan, as well as the drug being prescribed. Medicare, the federal health insurance program for people over 65 and certain individuals with disabilities, provides coverage for diabetes drugs for eligible beneficiaries. Medicare coverage for diabetes drugs can depend on the specific drug being prescribed, as well as the patient's insurance plan.
Medicaid, the joint federal-state program that provides health coverage for low-income individuals, also provides coverage for diabetes drugs. Medicaid coverage for diabetes drugs can vary depending on the specific drug being prescribed, as well as the state in which the patient resides. In addition to insurance coverage, there are also programs that provide financial assistance to patients who need help paying for their diabetes medications. For example, some pharmaceutical companies offer patient assistance programs that provide free or discounted medications to eligible patients.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Diabetes Drugs Market Segmentation
By Drug Type (Revenue, USD Bn):
The drug types considered, in this report include Injectable Drugs and Oral Drugs. Injectable drugs are further classified into insulin-based and non-insulin-based injectables. Oral drugs are further classified into various classes as per their mechanism of action as mentioned below :
- Injectable Drugs
- Insulin Based Injectables
- Non-insulin Based Injectables
- Exenatide (Byetta)
- Dulaglutide (Trulicity)
- Semaglutide (Ozempic, Wegovy)
- Liraglutide (`Saxenda, Victoza)
- Lixisenatide (Adlyxin)
- Pramlintide (Symlin)
- Tirzepatide (Mounjaro)
- Albiglutide (Tanzeum)
- Oral Drugs
- Biguanides - Metformin (Glucophage and Glucophase XR)
- Sulfonylureas - Glimepiride (Amaryl), Glyburide (DiaBeta), Glipizide (Glucotrol), Gliclazide (Diamicron), Chlorpropamide (Diabinese)
- Meglitinides and D-Phenylalanine Derivatives - Repaglinide (Prandin), Nateglinide (Starlix)
- Thiazolidinediones (TZDs) - Rosiglitazone (Avandia), Pioglitazone (Actos)
- Dipeptidyl peptidase-IV (DPP-4) inhibitors - Sitagliptin (Januvia), Saxagliptin (Onglyza), Linagliptin (Tradjenta), and Alogliptin (Nesina and Vipidia), Teneligliptin (Tenelia), Vildagliptin (Galvus)
- Alpha-glucosidase Inhibitors - Acarbose (Precose), Miglitol (Glyset), Voglibose (Voglib)
- Sodium-glucose co-transporter-2 (SGLT2) inhibitors - Canagliflozin (Invokana), Dapagliflozin (Farxiga), Empagliflozin (Jardiance), Ertugliflozin (Stelgatro)
- Dopamine D2 agonist – Bromocriptine (Parlodel and Cycloset)
- Glucagon like peptide 1 (GLP-1) receptor agonists - Semaglutide (Rybelsus)
- Bile Acid Sequestrants (BASs) - Colesevelam (Welchol)
- Others (Fixed Dose Combination Drugs)
By Application (Revenue, USD Bn):
Based on application, the market is segmented into Type 1 and Type 2. The 2 types of diabetes drugs are segmented and dominate the market. The Type 2 diabetes segment accounts for the largest sales of the worldwide market a few different kinds. The excessive prevalence of type 2 because of sedentary lifestyles and obesity in all age groups is attributed to the current situation. Around 10% of all diabetes cases are type 1, and approximately 90% of all cases of diabetes in UK are type 2. Hence, it is estimated to the diabetes drugs market will grow across the globe during the forecast period.
- Type 1 diabetes (due to β-cell destruction, usually leading to absolute insulin deficiency)
- Type 2 diabetes (due to a progressive insulin secretory defect on the background of insulin resistance)
- Other diabetes types
By Distribution Channel (Revenue, USD Bn):
Based on distribution channels, the market is classified into hospital pharmacies, rental pharmacies, and online pharmacies. The hospital pharmacies captured the highest market share, owing to the availability of trained & qualified personnel and favorable reimbursement structure. Online pharmacies are estimated to register the highest CAGR in the forecast period, it is attributed to the technological adaptation and acceptance of online pharmacies. Retail pharmacies showed a moderate market share improvement in the healthcare facilities in developing countries is anticipated to propel the popularity of retail pharmacies during the forecast period.
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































