US Breast Pump Market Report
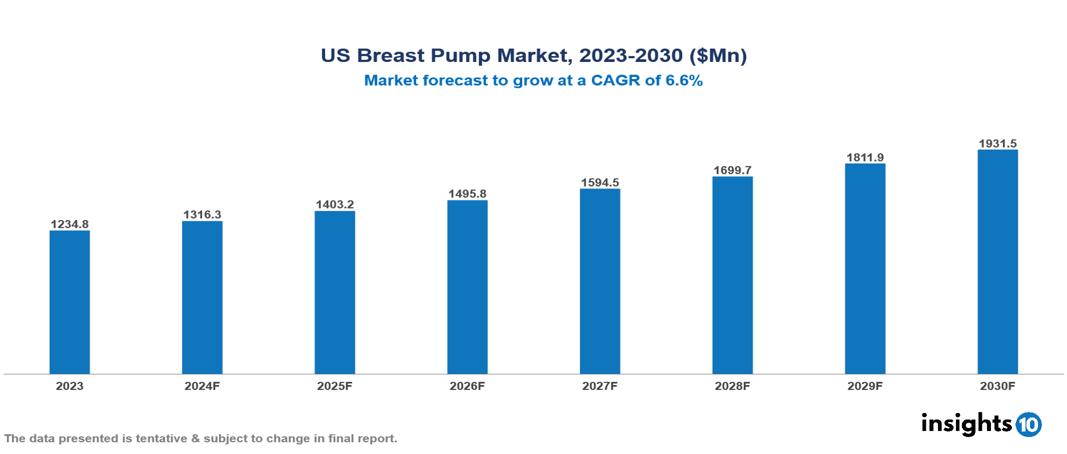
The US Breast Pump Market was valued at $1234.8 Mn in 2023 and is predicted to grow at a CAGR of 6.6% from 2023 to 2030, to $1931.5 Mn by 2030. The key drivers of the market include the rising female workforce, product innovations, and growing consumer awareness. The prominent players in the US Breast Pump Market are Magento Dr. Brown’s, Freemie, Evenflo, Nuk, Hygeia, Spectra Baby, Willow, Momcozy, and iAPOY, among others.
Buy Now

US Breast Pump Market Executive Summary
The US Breast Pump Market is at around $1234.8 Mn in 2023 and is projected to reach $1931.5 Mn in 2030, exhibiting a CAGR of 6.6% during the forecast period.
A breast pump is a mechanical device that lactating women use to extract milk from their breasts. They are available in two forms; they can be manual devices powered by hand or foot movements or automatic devices powered by electricity. Breast pumps are available in a variety of styles to meet the demands of moms. Hand-operated manual pumps are lightweight and silent, making them ideal for sporadic usage. Electric pumps are often used on a regular basis because of their higher efficiency and ability to run on mains or batteries. There are several uses for breast pumps. Many parents use them to continue breastfeeding after they return to work. They express their milk at work, which is later bottle-fed to their child by a caregiver. A breast pump may also be used to address a range of challenges parents may encounter during breastfeeding, including difficulties latching, separation from an infant in intensive care, feeding an infant who cannot extract sufficient milk itself from the breast, to avoid passing medication through breast milk to the baby, or to relieve engorgement, which is a painful condition whereby the breasts are overfull.
The US Breast Pump Market is driven by significant factors such as the rising female workforce, product innovations, and growing consumer awareness. However, stringent regulations, physical discomfort, stigma, and embarrassment restrict the growth and potential of the market.
The major players in the US Breast Pump Market are Magento Dr. Brown’s, Freemie, Evenflo, Nuk, Hygeia, Spectra Baby, Willow, Momcozy, and iAPOY, among others. Ameda, a part of Magento, Inc., is a leading manufacturer of breast pumps known for their innovative designs and efficient performance. Their pumps are designed to mimic natural breastfeeding patterns for optimal milk expression.
Market Dynamics
Market Growth Drivers
Rising Female Workforce: In 2023, women comprised 47% of the U.S. civilian labor force, a significant increase from 30% in 1950. As more women balance careers with motherhood, the demand for convenient breastfeeding solutions that fit their professional lives is growing. Breast pumps are becoming essential for working mothers, enabling them to continue breastfeeding while meeting work commitments. This rising need for breastfeeding-friendly solutions is driving the market for breast pumps and related products.
Product Innovations: Advances in breast pumps such as wearable, hands-free models, improved suction technologies, and smart features like app connectivity have significantly enhanced user convenience and comfort. These continuous improvements not only address the evolving needs of breastfeeding mothers but also stimulate market growth by attracting consumers seeking the latest and most efficient solutions for breastfeeding.
Growing consumer awareness: As information about the advantages of breastfeeding and the convenience of breast pumps becomes more widely available through healthcare professionals, online resources, and social media, more mothers are opting for these devices. Support from health organizations and government initiatives advocating for breastfeeding also contributes to this heightened awareness. Furthermore, the demand for adaptable parenting solutions and workplace accommodations for breastfeeding mothers has led to higher adoption of breast pumps, further fuelling market growth.
Market Restraints
Stringent Regulations: The US (Food and Drugs Administration) FDA enforces strict standards to guarantee the safety, effectiveness, and quality of breast pumps. These regulations demand comprehensive testing, certification, and documentation, which can be both expensive and time-consuming for manufacturers. Additionally, dealing with varying regulatory requirements across international markets adds further complexity. This increased regulatory burden can delay product introductions, restrict market access for smaller companies, and potentially raise prices for consumers, which can slow down market growth.
Physical Discomfort: The use of breast pumps often results in physical discomfort, such as sore and cracked nipples, pain from incorrect flange sizes or excessive suction, and hand fatigue from manual pumps. These issues can greatly affect a mother's pumping experience, making it crucial to choose the right pump, ensure proper fit, and use pain management techniques. These physical challenges contribute to an overall reduction in market growth.
Stigma and Embarrassment: Negative social attitudes and stigma related to breast pump usage can impede the growth of the breast pump market. Cultural and social biases against breastfeeding and breast pump use may deter some women from using these devices, particularly in public or professional settings. Concerns about the visibility of the pump, fear of judgment, and inadequate support from employers and society further contribute to this reluctance. These societal obstacles can hinder the broader acceptance and adoption of breast pumps, potentially restricting market expansion.
Regulatory Landscape and Reimbursement Scenario
The United States Food and Drug Administration (USFDA) is a federal agency of the Department of Health and Human Services. The main goal of the USFDA is to protect public health by ensuring the safety and efficacy of drugs, biological products, and medical equipment, along with food, cosmetics, and items that emit radiation. The Federal Food, Drug, and Cosmetic Act and several other comparable statutes are enforced by the FDA.
The process of gaining approval of new drugs involves rigorous and cumbersome evaluation by the USFDA. The two divisions of the USFDA, the Centre for Drug Evaluation and Research (CDER) and the Centre for Biologics Evaluation and Research (CBER) extensively review applications for approval which makes it challenging for pharmaceutical companies to bring a new product to the market. The various types of applications submitted to the USFDA for getting approval for pharmaceuticals include Investigational New Drug Application (INDA), New Drug Application (NDA), Abbreviated New Drug Application (ANDA) for generic drugs, and Biologics License Application (BLA).
Medicare and Medicaid are two of the health insurance programs administered by the US Government, with differences in covered services and cost-sharing. Medicare is a government health insurance program available to Americans 65 years of age or older, as well as to younger individuals with disabilities. Medicaid provides health coverage to low-income families, based on eligibility determined by comparing the income with the federal poverty level. Initially launched in 1965 by the Social Security Administration, the Centres for Medicare and Medicaid Services (CMS) currently oversee its administration. Medicare and Medicaid thus provide financial security lessen the financial burden of healthcare and provide better outcomes for beneficiaries.
Competitive Landscape
Key Players
Here are some of the major key players in the US Breast Pump Market:
- Magento (Ameda)
- Dr. Brown’s
- Freemie
- Evenflo
- Nuk
- Hygeia
- Spectra Baby
- Willow
- Momcozy
- iAPOY
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Breast Pump Market Segmentation
By Product Type
- Manual pumps
- Battery-powered pumps
- Electric pumps
By Pump System
- Open System
- Closed System
By Pumping Type
- Single
- Double
By Distribution Channel
- Hospital Pharmacies
- Retail Stores
- Online Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































