US Biobanks Market Analysis
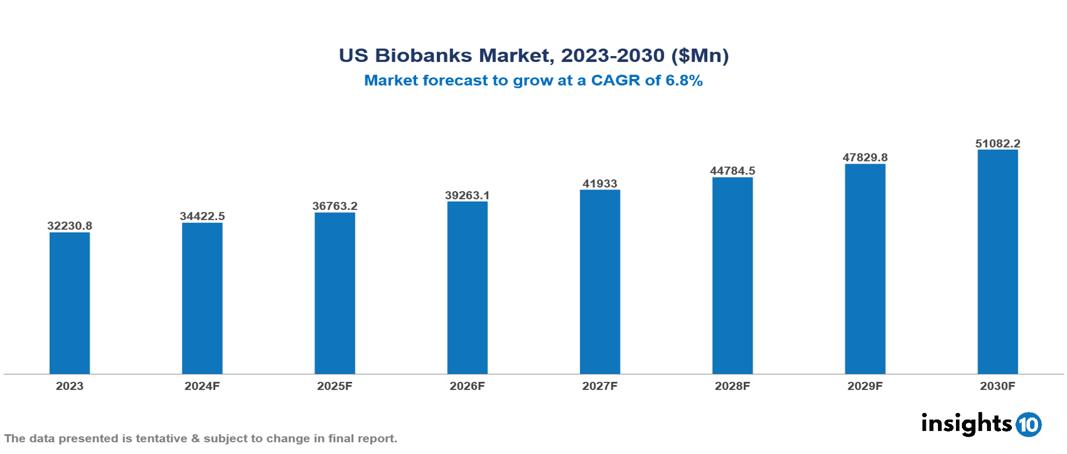
The US Biobanks Market was valued at $32,230.80 Mn in 2023 and is predicted to grow at a CAGR of 6.80% from 2023 to 2030, to $51,082.23 Mn by 2030. The key drivers of this industry include government initiatives, growing demand for human samples, and the prevalence of chronic diseases. The industry is primarily dominated by players such as Thermo Fisher Scientific, Merck KGaA, QIAGEN, and Tecan Group among others.
Buy Now

US Biobanks Market Executive Summary
The US Biobanks Market was valued at $32,230.80 Mn in 2023 and is predicted to grow at a CAGR of 6.80% from 2023 to 2030, to $51,082.23 Mn by 2030.
A biobank is a carefully curated collection of primarily human biological samples and associated data, systematically organized for research purposes. These facilities are pivotal in medical research, aiding diverse studies such as genomics and personalized medicine. Each biobank collects samples and data tailored to its specific research goals. For example, some focus on diseases like cancer, while others gather samples and data from specific demographics or geographic regions.
Globally, the majority of biobanks are located in North America and Europe, which together account for about 95 percent of all biobanks. Research related to biobanks predominantly focuses on a few disease areas, such as obesity, Alzheimer's, breast cancer, and diabetes, comprising 20% of all publications in the field. The market is driven by significant factors like government initiatives, growing demand for human samples, and the prevalence of chronic diseases. However, ethical and legal considerations, data management and integration, and public trust and participation restrict the growth and potential of the market.
Prominent players in this field include Thermo Fisher Scientific, Merck KGaA, QIAGEN, and Tecan Group among others.
Market Dynamics
Market Growth Drivers
Government Initiatives: Robust government backing, including funding and initiatives like the National Institutes of Health (NIH) and the All of Us Research Program, promotes precision medicine. This support is a market driver for the biobank market in the US, as it enhances research capabilities and fosters the development of personalized treatment approaches.
Growing Demand for Human Samples: The growing demand for human samples like blood, plasma, serum, and urine for drug development, cancer research, and studies on respiratory diseases and Alzheimer's disease drives the need for biobanks. This demand is a market driver for the biobank market in the US, as it underscores the critical role biobanks play in supporting advanced medical research and therapeutic development.
Prevalence of Chronic Disease: According to the US Department of Health and Human Services, around 129 Mn people in the US suffer from at least one major chronic disease. This prevalence is a market driver for the biobank market in the US, as it increases the demand for research and development of treatments, supported by biobank resources.
Market Restraints
Ethical and Legal Considerations: Strict regulations and ethical guidelines concerning consent, privacy, and data security present a market restraint for the biobank market in the US. These requirements can create operational complexities and potentially limit the scope of sample collection, impacting the biobanks' ability to gather and utilize biological specimens effectively for research and development purposes.
Data Management and Integration: Handling extensive datasets and integrating varied data sources present technical hurdles, necessitating robust infrastructure and specialized expertise. This complexity acts as a market restraint for the biobank sector in the US, as it requires substantial resources and capabilities to manage data effectively, potentially limiting operational efficiency and research outcomes.
Public Trust and Participation: Fostering public trust and increasing broad participation in biobank initiatives can be challenging due to apprehensions regarding data privacy and the utilization of biological samples. This represents a market restraint for the biobank market in the US, as addressing these concerns effectively is crucial for sustaining public support and participation in research endeavors.
Regulatory Landscape and Reimbursement Scenario
The Food and Drug Administration (FDA) oversees the handling of human tissues and cells used in medical products, ensuring their proper collection, storage, and distribution. The Health Insurance Portability and Accountability Act (HIPAA) establishes federal standards to safeguard sensitive patient health information. Additionally, the Common Rule comprises federal regulations governing research involving human subjects, including studies using biospecimens and data from biobanks. Compliance with these regulations mandates institutional review board (IRB) approval for such research to protect participants' rights and ensure ethical conduct.
Competitive Landscape
Key Players
Here are some of the major key players in the US Biobanks Market:
- Thermo Fisher Scientific
- Merck KGaA
- QIAGEN
- Tecan Group
- Becton, Dickinson and Company
- STEMCELL Technologies Inc.
- Avantor
- BioLife Solutions
- Hamilton Company
- Azenta Life Science
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Biobanks Market Segmentation
By Product and Service
- Equipment
- Consumables
- Services
By Sample Type
- Blood Products
- Human Tissues
- Nucleic Acids
- Others
By Application
- Regenerative Medicine
- Life Science Research
- Clinical Research
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































