US Antifungal Drugs Market Analysis
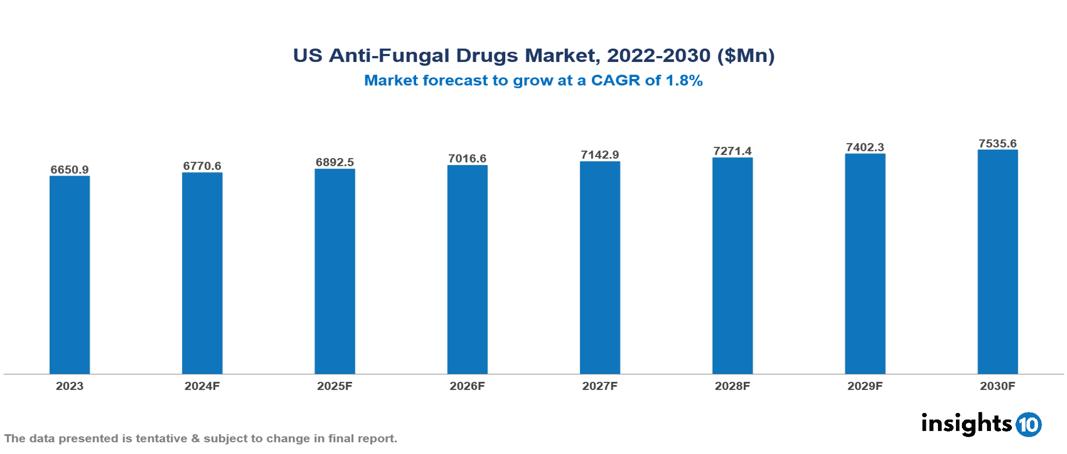
US Antifungal Drugs Market is at around $6.64 Bn in 2023 and is projected to reach $7.52 Bn in 2030, exhibiting a CAGR of 1.8% during the forecast period. The market is being driven by rising rates of fungal infections, improvements in drug development, and increase in antifungal resistance. The market is dominated by key players like Pfizer Inc., Merck & Co., Inc., Astellas Pharma Inc., Abbott Laboratories, GlaxoSmithKline plc, Bayer AG, Novartis AG, Glenmark Pharmaceuticals Limited, Sanofi, and Enzon Pharmaceuticals, Inc.
Buy Now

US Antifungal Drugs Market Executive Summary
US Antifungal Drugs Market is at around $6.64 Bn in 2023 and is projected to reach $7.52 Bn in 2030, exhibiting a CAGR of 1.8% during the forecast period.
The creation, manufacturing, and distribution of pharmaceuticals intended to eradicate fungal pathogens with the least amount of host toxicity is the industry that makes up the US Antifungal Drugs market. These medications address a range of fungal infections, and there are several kinds of antifungal medicines on the market, including azoles and polyenes. Drug resistance, the need for a wider range of antifungals to treat different fungal diseases, and the need for quicker and more potent medications are some of the factors affecting the market.
The growing prevalence of fungal diseases, technology breakthroughs, and the adoption of economical and effective manufacturing techniques have driven a revolutionary transformation in this industry.is fueling the steady rise of the US antifungal drug market. To expand the market, major players are focusing on research and development for novel therapies. Improvements in healthcare infrastructure and growing public knowledge of fungal infections contribute to the market's upward trend.
The market for antifungal drugs has grown at an impressive rate in recent years, and in 2023 it was valued at $15.8 Bn globally. Advances in treatment efficacy and accessibility have led to a significant change in the antifungal pharmaceutical landscape. Furthermore, the market for antifungal medications has seen significant investments, which have been crucial in promoting the industry's upward growth trajectory. This industry's ability to innovate, produce goods with greater efficiency, and enhance its finances highlights how dynamic and promising the antifungal drugs market is.
Amplyx Pharmaceuticals was acquired by Pfizer in 2021. Pfizer now has access to fosmanogepix, the principal antifungal medication candidate developed by Amplyx, which is undergoing Phase 2 clinical trials. With its distinct mode of action, fosmanogepix may be able to overcome some of the drawbacks of the antifungal medications that are now on the market. Pfizer is also creating its line of antifungal medications. Although these medications are still in the early phases of research and development, they may be able to fill gaps in the antifungal market's medical demands.
Market Dynamics
Market Growth Drivers:
Growing Prevalence of Fungal Infections: There is a growing need for antifungal medications as the number of fungal infections increases. Fungal diseases result in about 75,000 hospital admissions and almost 9 Mn outpatient visits annually in the US.
Developments in Drug Discovery: The pharmaceutical industry's ongoing research and development initiatives result in the release of novel, more potent antifungal medications. Market expansion is fueled by advancements in medication formulations, delivery systems, and therapy modalities.
Antifungal Resistance: New medications or combination therapies that can successfully combat resistant strains of the disease are being developed in response to the alarming growth of antifungal resistance. This spurs market growth and innovation.
Market Restraints:
High Treatment Costs: Certain patient populations may not be able to afford antifungal drugs, which could restrict their access to treatment and thus hamper the growth of the market.
Side Effects and Toxicity: Antifungal medications can occasionally have negative side effects and toxicity, which can force patients to stop taking them altogether.
Stringent Regulations: Getting regulatory approval for new antifungal medications can be a time-consuming and expensive procedure as the regulatory environment for drug approval and market entry is stringent.
Notable Recent Updates
- October 2023, the US Food and Drug Administration (FDA) permitted Lupin Ltd. to start selling its generic Fluconazole tablets, which are used to treat fungal infections
Healthcare Policies and Regulatory Landscape
The regulatory body in charge of overseeing pharmaceuticals in the US is the Food and Drug Administration (FDA). The FDA ensures that new drugs are safe and effective by evaluating and approving them. Pre-clinical, clinical, and FDA review are the three primary phases of the development process for new therapeutic drugs and biologics. The process of obtaining a license for a pharmaceutical involves several difficulties, including stringent rules, costly and time-consuming development schedules, and difficulties with clinical trials.
Competitive Landscape
Key Players:
- Pfizer Inc.
- Merck & Co., Inc.
- Astellas Pharma Inc.
- Abbott Laboratories
- GlaxoSmithKline plc
- Bayer AG
- Novartis AG
- Glenmark Pharmaceuticals Limited
- Sanofi
- Enzon Pharmaceuticals, Inc.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Antifungal Drugs Market Segmentation
By Drug Class
- Azoles
- Echinocandins
- Polyenes
- Allylamines
- Others
By Drug Indication
- Dermatophytosis
- Aspergillosis
- Candidiasis
- Others
By Drug Dosage
- Oral drugs
- Ointments
- Powders
- Others
By Infection
- Systemic Antifungal Infections
- Superficial Antifungal Infections
By Drug Distribution
- Hospital Pharmacies
- Retail Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































