US Advanced Therapy Medicinal Products (ATMPs) Market Analysis
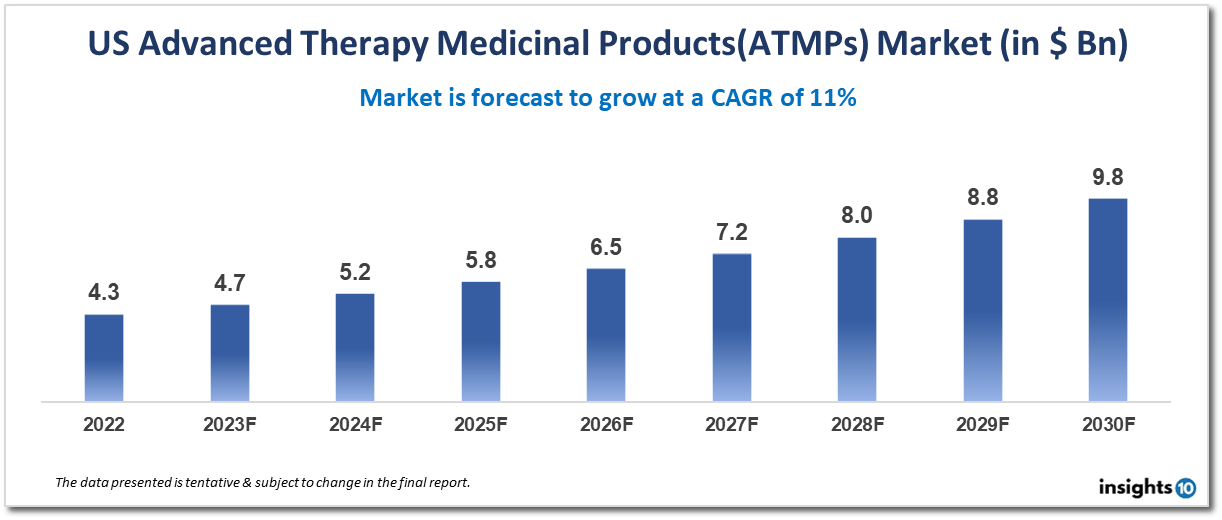
The US Advanced Therapy Medicinal Products (ATMPs) market is projected to grow from $4.3 Bn in 2022 to $9.8 Bn by 2030, registering a CAGR of 11% during the forecast period of 2022-30. One of the key drivers of growth in the US ATMP market is the increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular diseases. These diseases often require long-term treatment, and ATMPs offer a promising new approach to treating them. Additionally, advances in gene editing technologies such as CRISPR/Cas9 have increased the potential for ATMPs to cure genetic disorders. Some of the major players are Novartis International AG, Gilead Sciences, Inc., Bluebird Bio, Inc., Spark Therapeutics, Inc., and Kite Pharma, Inc.
Buy Now

US Advanced Therapy Medicinal Products (ATMPs) Market Executive Summary
It is anticipated that the US Advanced Therapy Medicinal Products (ATMPs) market will increase from $4.3 Bn in 2022 to $9.8 Bn by 2030, recording a CAGR of 11% over the forecast period of 2022-30.
Innovative therapies known as advanced therapy medicinal products (ATMPs) employ living cells, tissues, or genes to treat or cure illness. Due to the fact that they provide a fresh approach to treating complicated and life-threatening illnesses, these treatments are gaining popularity in the US. One of the major markets for ATMPs in the world, the US market is anticipated to expand dramatically over the coming few years.
The rising incidence of chronic illnesses including cancer, diabetes, and cardiovascular diseases is one of the main factors boosting the US ATMPs market. ATMPs provide a promising novel therapeutic strategy for the treatment of many disorders, which frequently require long-term care. Likewise, the potential for ATMPs to treat genetic abnormalities has expanded as a result of developments in gene editing tools like CRISPR/Cas9.
By establishing a regulatory environment that promotes the development of these treatments, the US Food and Drug Administration (FDA) has also significantly contributed to the expansion of the ATMPs industry. The FDA has expedited the approval procedure for these treatments and created a specialized office to supervise the creation and approval of ATMPs.
The US ATMPs market is divided into 3 product categories:
- Tissue engineering
- Cell therapy, and
- Gene therapy
The largest category is gene therapy, which has the ability to treat genetic illnesses by changing or replacing defective genes. Cell therapy, which uses live cells to replace or repair damaged tissues, is another important subsegment.
Notwithstanding the potential advantages of ATMPs, the US market faces a number of difficulties. Several of these medicines require specific manufacturing procedures, and their development is frequently time-consuming and costly. Access to these treatments may also be hampered by their high cost, and certain ATMPs' long-term effectiveness and safety have been questioned.
As these novel medicines provide a fresh approach to treating complicated and life-threatening illnesses, the US ATMPs market is anticipated to expand significantly over the next years. To guarantee the long-term viability of these medicines, the industry will need to solve a number of issues.
Market Dynamics
Market Growth Drivers
- Chronic diseases are becoming more common: One of the main factors propelling the market for ATMPs is the increased incidence of chronic illnesses including cancer, diabetes, and cardiovascular conditions. A potential new method of treating these disorders is provided by ATMPs.
- Improvements in gene editing technologies: The market is expanding as a result of the greater potential for ATMPs to treat genetic illnesses brought about by advances in gene editing technologies like CRISPR/Cas9
- Support from the FDA: The FDA has created a legal framework that promotes the creation of ATMPs, which is fueling the market expansion
- Rising R&D expenditures: The market is anticipated to rise as a result of rising R&D expenditures on ATMPs
Competitive Landscape
Key Players
- Novartis International AG
- Gilead Sciences, Inc.
- Bluebird Bio, Inc.
- Spark Therapeutics, Inc.
- Kite Pharma, Inc.
- Celgene Corporation
- Editas Medicine, Inc.
- Sangamo Therapeutics, Inc.
- Intellia Therapeutics, Inc.
- CRISPR Therapeutics AG
These companies are among the leading developers and manufacturers of advanced therapy medicinal products in the United States. They are involved in the development of innovative treatments for a variety of diseases and conditions using cutting-edge technologies such as gene therapy, cell therapy, and gene editing. These companies are also investing heavily in research and development to bring new treatments to market and meet the growing demand for advanced therapies
Healthcare Policies and Regulatory Landscape
The American healthcare regulatory and policy environment is complicated and ever-changing. The following are some crucial elements of the healthcare policy and regulatory environment.
- Affordable Care Act (ACA): To increase access to healthcare and lower healthcare costs, the Affordable Care Act, popularly known as Obamacare, was passed in 2010. It contains clauses like the individual mandate, which makes it mandatory for people to obtain health insurance and the creation of health insurance marketplaces.
- Both Medicaid and Medicare Millions of Americans are covered by the government-funded healthcare programs Medicare and Medicaid. Although Medicaid covers low-income individuals and families, Medicare covers the elderly and those with certain impairments.
- FDA guidelines: To assure their efficacy and safety, the FDA oversees pharmaceuticals, medical devices, and other healthcare items. Obtaining FDA clearance might take a while and be expensive, but it is required to launch innovative medications.
- The Health Insurance Portability and Accountability Act (HIPAA), which was passed in 1996, establishes guidelines for safeguarding the confidentiality and privacy of patient health information. Moreover, it has clauses that address patient notification in the case of a data breach.
- Value-based care is a type of healthcare delivery that places an emphasis on enhancing patient outcomes while cutting costs. It entails leveraging data to enhance the delivery of care and matching provider incentives with patient results.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Advanced Therapy Medicinal Products (ATMPs) Market Segmentation
By Product Type (Revenue, USD Billion):
- Cell Therapy
- Gene Therapy
- CAR-T Therapy
- Tissue Engineered Product
By Disease Type (Revenue, USD Billion):
- Alzheimer's
- Cystic Fibrosis
- Muscular Dystrophies
- Hemophilia
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacy
- Drug Store
- Retail Store
- Online Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































