US ADHD (Attention Deficit Hyperactivity Disorder) Therapeutic Market Analysis
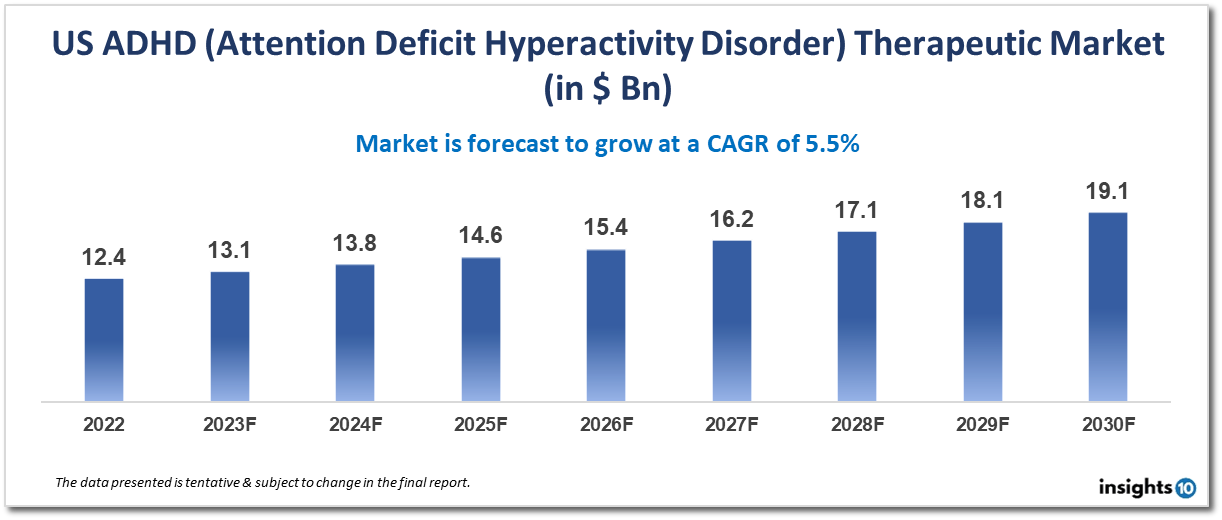
The US ADHD (Attention Deficit Hyperactivity Disorder) therapeutic market is projected to grow from $12.4 Bn in 2022 to $19.1 Bn by 2030, registering a CAGR of 5.5% during the forecast period of 2022-30. ADHD is one of the most common neurodevelopmental disorders in children, with an estimated prevalence of 6-9% in the US. The rising prevalence of ADHD is driving the demand for effective treatments and contributing to the growth of the market. Some key players in the US ADHD therapeutic market include Shire (now part of Takeda Pharmaceutical Company), Pfizer Inc., Novartis International AG, Johnson & Johnson, Eli Lilly and Company, and GlaxoSmithKline plc.
Buy Now

US ADHD (Attention Deficit Hyperactivity Disorder) Therapeutic Market Executive Summary
A CAGR of 5.5% is anticipated for the US ADHD (Attention Deficit Hyperactivity Disorder) treatment market from 2022 to 2030, growing from $12.4 Bn in 2022 to $19.1 Bn in 2030.
Due to the rising incidence of ADHD and the availability of efficient treatment alternatives, the US therapeutic market for ADHD (Attention Deficit Hyperactivity Disorder) is expanding quickly.
Treatment options for people with ADHD are referred to as ADHD (Attention Deficit Hyperactivity Disorder) Therapy. Inattention, hyperactivity, and impulsivity are some of the symptoms of ADHD, a neurodevelopmental condition. While it is frequently identified in children, ADHD can last until adulthood. Although the precise aetiology of ADHD is unknown, a confluence of genetic, environmental, and neurological factors is thought to be responsible.
Medication, behavioural therapy, and lifestyle changes are just a few of the therapies for ADHD that are available. Stimulants like methylphenidate and amphetamine are the most often recommended treatments for ADHD because they raise dopamine and norepinephrine levels in the brain. For people who do not respond well to stimulants or who have negative effects from them, non-stimulant medicines such as atomoxetine and guanfacine may be utilised.
For those with ADHD, particularly youngsters, behavioural treatment might be helpful. Working with a therapist or counsellor to develop behavioural methods and coping mechanisms that can help control ADHD symptoms is known as behavioural therapy. For those with ADHD, making lifestyle changes like exercising and adopting a nutritious diet may be helpful.
Market Dynamics
Market Growth Drivers
Due to the lack of a cure for this disorder, the prevalence of ADHD is increasing at a faster rate, which drives the US market for therapeutics for the condition. Additionally, the market for ADHD therapeutics is primarily driven by the negative effects of unstable lifestyles and food additives on children, which is increasing the prevalence of ADHD in people between the ages of 4 to 17. Additionally, it is predicted that in the near future, the US market for ADHD will experience revenue growth due to rising awareness of the syndrome among doctors and patients as well as opinion-based treatment options caused by the absence of routine diagnostic tests.
Market Restraints
The development of the US market for ADHD therapeutics is being constrained by the high cost of ADHD medications, poor reimbursement policies, and side effects associated with ADHD medications (such as insomnia, anorexia, dizziness, headaches, and mood changes). The adverse effects of ADHD medications also negatively impact patients' quality of life and restrain the market's revenue growth over the given forecast period. Aside from these issues, medications for attention deficit hyperactivity disorder are linked to medical conditions like kidney disease, coronary artery disease, and seizures.
Competitive Landscape
Key Players
- Shire (currently part of Takeda Pharmaceutical Company) (now part of Takeda Pharmaceutical Company)
- Pfizer, Inc.
- Incorporated by Novartis
- Smith & Johnson
- Firm named Eli Lilly
- PLC GlaxoSmithKline
- Pharmaceutical company Teva Industries Ltd.
- Impax Laboratories, LLC, Mylan N.V.
- Pharmaceutical company Mallinckrodt
These businesses also produce additional therapeutic goods including behavioural treatment programmes in addition to a variety of ADHD medicines, including stimulant and non-stimulant drugs. These businesses dominate the market and are responsible for generating both innovation and competition. The development of novel ADHD therapies and technology by several smaller businesses and startups may potentially have an impact on the market in the future.
Healthcare Policies and Regulatory Landscape
The US Food and Drug Administration (FDA) regulates the US ADHD treatment market, which is also subject to a variety of healthcare laws and regulations. Following are some significant laws and rules that affect the market:
- FDA Approval: Before being marketed and sold in the US, all ADHD drugs must have FDA approval. The FDA evaluates evidence from clinical trials and other sources to assess the effectiveness and safety of each drug.
- The Drug Enforcement Agency (DEA) has classed certain ADHD drugs as Schedule II prohibited narcotics due to their propensity for misuse and dependency. The prescribing and distribution of certain pharmaceuticals are subject to extra rules as a result of this categorization.
- Medicaid Coverage: ADHD medicines and other therapeutic interventions are covered by Medicaid for those who qualify, which has a big influence on the market demand for these items
- Healthcare insurers are required to cover mental health problems, including ADHD, under the requirements of the Affordable Care Act (ACA). For people with ADHD, this has boosted access to treatment alternatives.
- Guidelines for Diagnosis and Treatment: For the diagnosis and management of ADHD, the American Academy of Pediatrics and other medical associations have issued recommendations. These recommendations for best practices might have an effect on how ADHD drugs are prescribed and used.
- Adverse Event Reporting: The FDA requires ADHD drug manufacturers to notify it of any adverse events or side effects related to their medicines. This enables continuous monitoring and evaluation of the risks and advantages of certain treatments, helping to assure their safety.
Overall, these laws and rules are crucial in establishing the US ADHD therapeutic market, guaranteeing the effectiveness and safety of ADHD drugs and other therapies, and facilitating access to healthcare for people with ADHD.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US ADHD (Attention Deficit Hyperactivity Disorder) Therapeutic Market Segmentation
By Drug Type (Revenue, USD Billion):
- Stimulants
- Amphetamine
- Methylphenidate
- Dextroamphetamine
- Dexmethylphenidate
- Lisdexamfetamine
- Others
- Non-Stimulants
- Atomoxetine
- Bupropion
- Guanfacine
- Clonidine
By Age Group (Revenue, USD Billion):
- Pediatric And Adolescent
- Adult
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Speciality Clinics
- Retail Pharmacies
- e-Commerce
By Psychotherapy (Revenue, USD Billion):
- Behaviour Therapy
- Cognitive Behavioral Therapy
- Interpersonal Psychotherapy
- Family Therapy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































