UK Infectious Disease Therapeutics Market Analysis
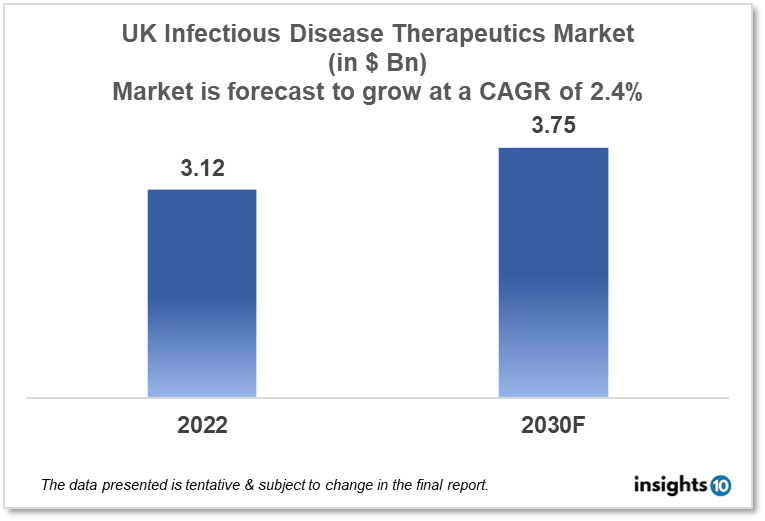
By 2030, it is anticipated that the UK infectious disease therapeutics market will reach a value of $3.75 Bn from $3.12 Bn in 2022, growing at a CAGR of 2.4% during 2022-2030. Infectious Disease Therapeutics in the UK is dominated by a few domestic pharmaceutical companies such as GlaxoSmithKline, Faron Pharmaceuticals and Emergent BioSolutions. The infectious disease therapeutics market in the UK is segmented into different therapeutic areas and different treatment types. The major factors affecting the UK infectious disease therapeutics market are the increasing disease burden of communicable diseases like TB, hepatitis, and COVID-19 and the lack of healthcare funding for infectious disease treatment in various areas of the UK.
Buy Now

UK Infectious Disease Therapeutics Analysis Summary
By 2030, it is anticipated that the UK infectious disease therapeutics market will reach a value of $3.75 Bn from $3.12 Bn in 2022, growing at a CAGR of 2.4% during 2022-2030.
The UK is a high-income, developed island country spanning an archipelago including Great Britain, located in Western Europe comprising England, Scotland, Wales, and Northern Ireland. Communicable diseases, often known as infectious diseases, are illnesses that can be passed from person to person directly or indirectly. Diseases such as measles or mumps can spread by coughing, sneezing or touching infected surfaces. Sexually transmitted diseases (STIs) and hepatitis are spread via bodily fluids like blood or sperm.
Several improvements in infectious disease therapies have occurred in recent years, including the creation of novel antibiotics and antiviral medications, as well as advances in immunotherapy and vaccine development. Immunotherapy is a new therapeutic approach that leverages the body's immune system to combat infectious diseases. The development of immunotherapies for diseases such as HIV and hepatitis C has received special attention in the UK. UK's government spends 12 % of its GDP on healthcare.
Market Dynamics
Market Growth Drivers Analysis
In response to the COVID-19 pandemic, the UK has made significant investments in the development and manufacturing of COVID-19 vaccines and therapies. Several medications, including remdesivir, dexamethasone, and tocilizumab, have been authorised for use in the UK to treat COVID-19. The Small Business Research Initiative (SBRI) and the Longitude Prize are two initiatives sponsored by the UK government to assist in the development of novel antibiotics. The UK has been involved in the development of various novel malaria treatments. Tafenoquine, a novel medicine that has been found to be effective against malaria and has recently been approved for use in the UK, is one example. Cutting-edge pharmaceuticals sectors in the UK give a push to the expansion. These aspects could boost UK infectious disease therapeutics market.
Market Restraints
The UK's low productivity and training deficit are not favourable to innovation. Developing new pharmaceuticals and treatments necessitates adhering to regulatory rules established by organisations such as the UK's Medicines and Healthcare products Regulatory Agency (MHRA). These requirements can be complicated and time-consuming, resulting in delays and higher expenses. These factors may deter new entrants into the UK infectious disease therapeutics market.
Competitive Landscape
Key Players
- GlaxoSmithKline (GSK) - GSK is a major British pharmaceutical corporation. GSK has collaborated with other firms to develop and manufacture vaccines and therapies in response to the COVID-19 pandemic
- AstraZeneca - AstraZeneca is another major British pharmaceutical company that develops and manufactures infectious disease therapeutics. During the COVID-19 pandemic, AstraZeneca partnered with the University of Oxford to develop and manufacture a vaccine
- Oxford BioMedica - Oxford BioMedica is a British biopharmaceutical company that has been involved in the development of several vaccines and treatments for infectious diseases, including COVID-19
- Faron Pharmaceuticals - Faron Pharmaceuticals is a British biopharmaceutical company, that has a lead product, Traumakine, which is a potential treatment for COVID-19-induced acute respiratory distress syndrome
- Emergent BioSolutions - Emergent BioSolutions is a multinational biopharmaceutical company which develops and manufactures a range of infectious disease therapeutics, including vaccines and treatments for anthrax, smallpox, and botulism
Recent Notable Updates
December 2022: Following the European Commission's licence in November 2022, GSK has announced that the UK's Medicines and Healthcare products Regulatory Agency (MHRA) has approved Sanofi's SARS-CoV-2 spike protein (B.1.351 strain) vaccine for the prevention of COVID-19 disease in adults aged 18 and above in Great Britain.
Healthcare Policies and Reimbursement Scenarios
In the UK, the regulation of infectious disease therapeutics is primarily overseen by the Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA is in charge of guaranteeing the safety, effectiveness, and high quality of all medicines and medical equipment, including those used to treat infectious diseases. In order to be licenced for use in the UK, infectious disease treatments must first go through a rigorous regulatory process that includes clinical studies and other testing to ensure their safety and efficacy. Following the approval of a product, the MHRA continues to monitor its safety and quality through post-marketing surveillance. Other UK regulatory bodies that may be involved in the regulation of infectious disease therapeutics, in addition to the MHRA, include the National Institute for Health and Care Excellence (NICE), which provides guidance on the use of new treatments within the NHS, and the Health and Safety Executive (HSE), which regulates the use of hazardous substances in the workplace.
In the UK, the National Health Service (NHS) manages the reimbursement of infectious disease medicines through a system known as the National Institute for Health and Care Excellence (NICE). NICE assesses new medications and therapies for efficacy, safety, and cost-effectiveness before recommending whether they should be paid for by the NHS.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Infectious Disease Therapeutics Segmentation
By Mode of Treatment (Revenue, USD Billion):
- Vaccines
- Drugs
By Applications (Revenue, USD Billion):
- HIV/AIDS
- Influenza
- Hepatitis
- Malaria
- Tuberculosis
- Others
By Disease Type (Revenue, USD Billion):
- Viral Diseases
- Bacterial Diseases
- Fungal Diseases
- Parasitic Diseases
- Others
By Target Organism (Revenue, USD Billion):
- Antibiotics
- Antivirals
- Antifungals
- Anti-Parasitic
- Others
By End User (Revenue, USD Billion):
- Hospitals and Clinics
- Ambulatory Care Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































