UK Hypersomnia Therapeutics Market Analysis
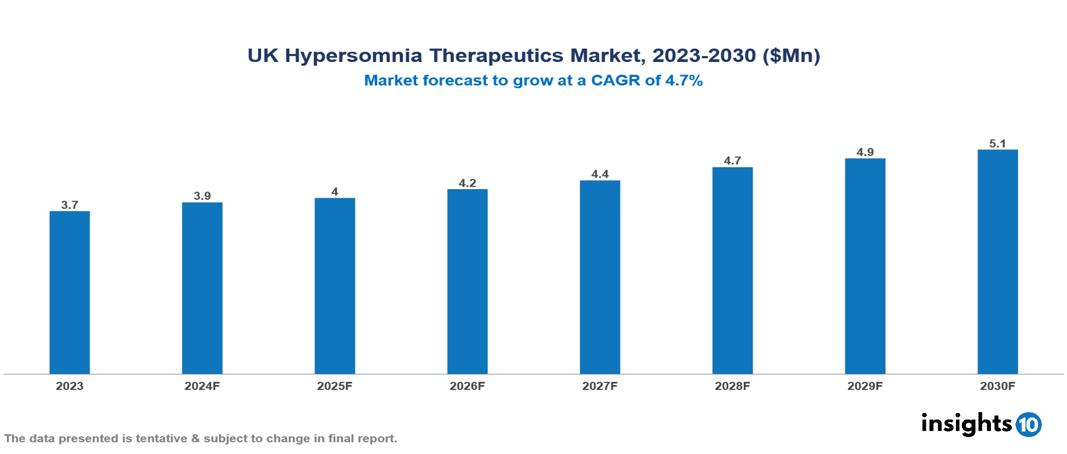
The UK Hypersomnia Therapeutics Market was valued at $3.70 Mn in 2023 and is predicted to grow at a CAGR of 4.70% from 2023 to 2030, to $5.10 Mn by 2030. The key drivers of this industry include increasing awareness, lifestyle changes, expanding geriatric population, and early detection. The key players in the industry are GSK, Hoffmann-La Roche Ltd, Sanofi, and Novartis among others.
Buy Now

UK Hypersomnia Therapeutics Market Executive Summary
The UK Hypersomnia Therapeutics Market is at around $3.70 Mn in 2023 and is projected to reach $5.10 Mn in 2030, exhibiting a CAGR of 4.70% during the forecast period.
Hypersomnia is a group of neurological disorders characterized by excessive daytime sleepiness, despite getting enough sleep at night. This can significantly impair daily functioning and reduce quality of life. While the exact causes are still being researched, hypersomnia can be linked to underlying medical conditions, neurological disorders, or disruptions in sleep regulation mechanisms. People with hypersomnia often experience symptoms such as not feeling refreshed upon waking, feeling groggy and slow for extended periods after waking, and an excessive need for sleep despite sleeping for long periods. Chronic headaches, loss of appetite, excessive sweating, and depression have also been reported. Treatment for hypersomnia focuses on managing daytime sleepiness. This can involve adopting healthy lifestyle practices like eating well and exercising regularly. Medications like stimulants can also be prescribed to promote wakefulness. In some cases, cognitive behavioral therapy for insomnia (CBT-I) can help address underlying sleep habits that contribute to hypersomnia.
The prevalence of hypersomnia among the general population is 0.3%. The market therefore is driven by significant factors like the increasing awareness about hypersomnia disorders, increasing aging population and growing demand for precision medicine. However, potential side effects, high development costs, and strict regulatory approval restrict the market growth
The leading pharmaceutical companies include GSK, Pfizer is known for its extensive portfolio of medications, including those used to treat hypersomnia. Sanofi, Novartis, and Roche are also significant contributors to the hypersomnia therapeutics landscape, with continuous research and development activities.
Market Dynamics
Market Growth Drivers
Increasing Awareness about Hypersomnia Disorders: Increasing public awareness programs and training of health professionals can make more accurate decisions and increase the number of patients for these drugs. Every Mind Matters is a government-backed initiative by Public Health England that contains information about sleep and improving sleep hygiene.
Expanding Geriatric Population: Older adults are more likely to experience hypersomnia, primarily because of alterations in their sleep patterns and underlying health conditions. Given that a substantial portion of the population comprises older individuals (19%), there is a projected rise in the prevalence of hypersomnia disorders, which will drive the demand for treatment options that are specifically designed to cater to the distinct requirements of this age group.
Growing Demand for Precision Medicine: The development of precision medicine and personalized treatments for hypersomnia is driving the growth of targeted therapies in the UK market. Researchers are investigating novel treatments like low-sodium oxybate, which has shown promise for long-term management of narcolepsy and idiopathic hypersomnia. However, the high costs of these medications can be a significant barrier to access for some patients.
Market Restraints
Strict Regulatory Approval Process: The regulatory landscape in UK is stringent, with comprehensive guidelines governing the approval and marketing of pharmaceuticals. This leads to prolonged approval processes for new hypersomnia drugs, potentially delaying their availability to patients and limiting treatment options.
High Development Cost: The costs associated with developing and utilizing hypersomnia drugs are substantially high. This financial burden restricts access to these therapies, especially for patients requiring long-term treatment, particularly in a healthcare system where budget constraints are common.
Potential Side Effects: Hypersomnia drugs, despite alleviating severe drowsiness, carry potential side effects. Some side effects discourage people from starting or continuing treatment. The quality of life for hypersomnia patients is negatively impacted, resulting in a challenge to market growth within the pharmaceutical industry.
Regulatory Landscape and Reimbursement Scenario
The Medicines and Healthcare Products Regulatory Agency (MHRA) is the UK government body responsible for regulating medicines, medical devices, and blood products. They provide guidance and support to companies developing new medicines and medical devices ensuring their safety, quality, and efficacy.
The MHRA utilizes an electronic Common Technical Document (eCTD) format for applications. It assembles a team of experts to rigorously review all submitted documents and data. The MHRA grants a Marketing Authorisation (MA) allowing the product to be marketed and sold in the UK. Pharmaceutical companies are obligated to submit periodic safety update reports to monitor the drug's performance and identify any potential long-term side effects.
The UK healthcare system relies heavily on the National Health Service (NHS), which offers a comprehensive range of medical services funded through taxation. It includes a range of diagnostic tests and procedures, medications prescribed by doctors, and consultations with general practitioners.
Competitive Landscape
Key Players
Here are some of the major key players in the UK Hypersomnia Therapeutics Market:
- Sanofi
- Novartis
- Hoffmann-La Roche Ltd
- Pfizer
- AstraZeneca
- Evox Therapeutics
- Merck & Co. Inc.
- Ascend Biopharma
- GlaxoSmithKline
- Theranexus
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UK Hypersomnia Therapeutics Market Segmentation
By Application
- Idiopathic Hypersomnia
- Narcolepsy Type-1
- Narcolepsy Type-2
By Product
- Anti-depressants
- Stimulants
- Sodium oxybate
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































