UK Hyperhidrosis Therapeutics Market Analysis
UK hyperhidrosis therapeutics market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023-30. The increase in disease incidence, particularly axillary hyperhidrosis in industrialised nations, is one of the causes driving the market for hyperhidrosis treatments. Additionally, it is projected that increasing healthcare service demand will considerably fuel market growth, along with an increase in new product releases and product approvals.The global hyperhidrosis treatment market?s major key players are AbbVie Inc. (Allergan PLC), Brickell Biotech Inc., Eli Lilly and Company (Dermira), 1315 Capital (miraDry Inc.), SweatBlock, Merz Pharma (Merz Aesthetics), Dermavant Sciences Inc. (Roivant Sciences), Advin Health Care, Dermadry Laboratories Inc., and Dermata Therapeutics Inc
Buy Now

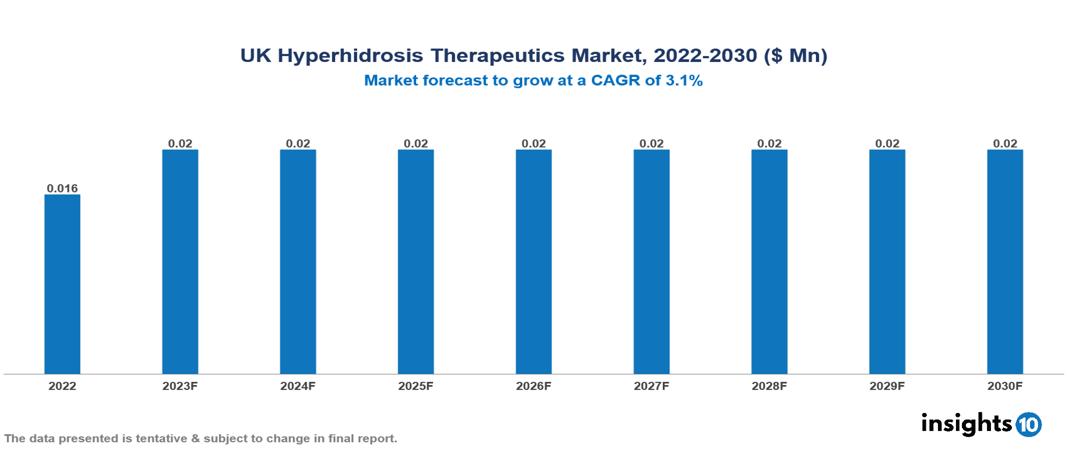
UK Hyperhidrosis Therapeutics Market Analysis Summary
UK Hyperhidrosis Therapeutics Market is valued at around $0.016 Mn in 2022 and is projected to reach $0.02 Mn by 2030, exhibiting a CAGR of 3.1% during the forecast period 2023-2030.
Primary hyperhidrosis is characterized by excessive amounts of perspiration that are concentrated in particular body parts, such as the palms, feet, and underarms. The market has been dominated by primary hyperhidrosis treatments, and this trend is predicted to continue. Most advanced treatments fall under the categories of topical and surgical treatments. The severity and cost of the condition determine the choice of therapy. The rise in secondary hyperhidrosis disorders and higher player investment in R&D are the main drivers of the hyperhidrosis therapy market growth. The market is expanding in the region for various reasons, such as the rising prevalence of secondary hyperhidrosis. The US has the highest proportion of hyperhidrosis patients among developed nations. Based on the sickness trends currently seen in the US, it is probable that the prevalence of hyperhidrosis will dramatically grow. Additionally, favorable reimbursement laws are expected to speed up industry expansion.
The market for treating hyperhidrosis is moderately consolidated with a few significant companies. Only a few of the top competitors presently control the majority of the market in terms of market share. Additionally, a number of big businesses are aggressively pursuing business deals with other corporations in an effort to strengthen their positions in the global market (Sientra acquired Miramar). Allergan, Brickell Biotech, Dermira, GlaxoSmithKline, Revance Therapeutics, Ulthera, TheraVida, and Sientra are a few of the global participants in this market.
Market Dynamics
Market Drivers
The condition known as secondary hyperhidrosis occurs when uncontrollable sweating is caused by an underlying medical condition. Excessive body sweating is more likely to be caused by the less common condition. The market for secondary hyperhidrosis therapy is growing as secondary hyperhidrosis prevalence rises. The 2020 International Hyperhidrosis Society estimates that 365 Mn people, or around 5% of the world's population, suffer from secondary hyperhidrosis and excessive sweating. In contrast to primary hyperhidrosis, which frequently manifests in childhood or adolescence, excessive sweating usually begins in adulthood. Adults are more prone to chronic illnesses as a result. The rapid rise of the adult population is causing a rapid increase in the prevalence of secondary hyperhidrosis.
Additionally, the journal predicts that there will be 643 Mn diabetics worldwide by 2030 and 783 Mn by 2045. According to this data, the prevalence of diabetes mellitus will rise concurrently with the incidence of secondary hyperhidrosis. Therefore, it is projected that these factors will boost demand for hyperhidrosis therapies, supporting the market's overall growth.
Market Restraints
The demand for hyperhidrosis treatment is constrained by the short-term nature of available treatments. A sympatheticectomy is an additional treatment for hyperhidrosis, which increases the risk of infection and a condition known as compensatory sweating. According to research published in 2022 by the American Academy of Dermatology Association, a botulinum toxin injection temporarily inhibits the body's chemicals from activating sweat glands. Four to five days into treatment, the majority of patients start to see improvement. According to the 2022 SAFELipo, BOTOX injections also stop sweat glands from secreting sweat. It is less dangerous than thermal liposuction. BOTOX is a temporary solution; to maintain the effects, continue the injections. According to the American Academy of Dermatology Association in 2022, a sympathectomy may potentially harm the nerves that connect the brain and eyes, cause dangerously low blood pressure, an irregular pulse, and reduce the body's capacity to resist heat. The short-term effects of cosmetic and therapeutic therapies are therefore expected to have a negative impact on the researched sector during the projected period.
Key players
Ipsen Novartis Pfizer Sanofi Shire BioMarin Pharmaceutical Takeda Pharmaceutical Company Intercept Pharmaceuticals Catalyst Pharmaceuticals Novo Nordisk1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UK Hyperhidrosis Therapeutics Market Segmentation
By Treatment:
- Topical Treatments
- Surgical Treatments
- Botulin toxin A
- Iontophoresis
- Laser Treatments
- Other Treatment Types
By Disease:
- Primary Focal Hyperhidrosis
- Secondary Focal Hyperhidrosis
- Generalized Hyperhidrosis
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































