UK Genomic Diagnostics Market Analysis
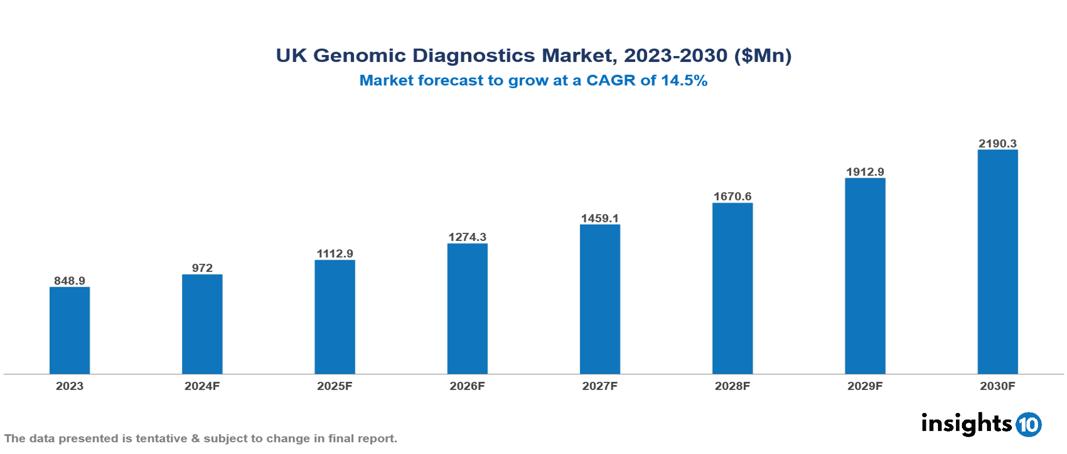
UK Genomic Diagnostics Market was valued at $848.90 Mn in 2023 and is predicted to grow at a CAGR of 14.5% from 2023 to 2030, to $2,190.26 Mn by 2030. The key drivers of this industry include rising disease incidence, increased government support, and growing public awareness. The industry is primarily dominated by Illumina, 23andMe, Myriad Genetics, and Amgen among others.
Buy Now

UK Genomic Diagnostics Market Executive Summary
UK Genomic Diagnostics Market was valued at $848.90 Mn in 2023 and is predicted to grow at a CAGR of 14.5% from 2023 to 2030, to $2,190.26 Mn by 2030.
Genomic diagnostics is a rapidly evolving field that uses an individual's genetic information to diagnose diseases, assess predisposition to future health problems, and guide treatment plans by analyzing DNA or RNA for disease-linked variations. This includes karyotyping to examine chromosome abnormalities, targeted mutation analysis for specific disease-related genes, and next-generation sequencing (NGS) for a comprehensive genetic analysis. Applications encompass disease diagnosis, carrier testing for informed family planning, predictive testing for disease risk assessment, and pharmacogenomics for personalized medication treatments. The benefits of genomic diagnostics include early disease detection, personalized medicine, and improved disease management and prognosis.
In the UK, chronic diseases are showing significant trends. Coronary heart disease (CHD) rates rise with age, with men experiencing three times the mortality rate compared to women, and a relatively low rate of 5.7 deaths per 100,000. Type 2 diabetes incidence and prevalence are expected to rise significantly by 2040, with mortality currently at half the incidence rate. Chronic obstructive pulmonary disease (COPD) is projected to increase by 37% by 2040, and cancer prevalence is expected to grow by 31%. Additionally, chronic pain affected 34% of those aged 16 and older and 53% of those aged 75 and older in 2017, while chronic kidney disease prevalence is set to rise by 34% by 2040.
Market is therefore driven by significant factors like rising disease incidence, increased government support, and growing public awareness. However, ethical and regulatory concerns, shortage of skilled professionals, and reimbursement challenges restrict the growth and potential of the market.
A prominent player in this field is Illumina, which has partnered with AstraZeneca to leverage genomics and AI for faster drug development by identifying new therapeutic targets and biomarkers, 23andMe acquired Lemonaid Health to enhance its personalized healthcare offerings through telehealth and prescription drug delivery services based on genetic information. Other contributors include Myriad Genetics, and Amgen among others.
Market Dynamics
Market Growth Drivers
Rising Disease Incidence: With approximately 360,000 new cancer cases annually in the UK, there is an increasing demand for advanced diagnostic tools. Genomic diagnostics offer the potential for earlier and more accurate diagnoses, driving their adoption in healthcare settings.
Increased Government Support: The UK government has demonstrated strong commitment to genomics with a $190Mn investment announced in December 2022. This funding is aimed at advancing genomic-based diagnostics for cancer and rare childhood diseases, promoting further research and development.
Growing Public Awareness: As public understanding of the genetic basis of diseases increases, there is greater demand for personalized medicine. Genomic diagnostics are crucial for tailoring treatments to individuals' genetic profiles, aligning with this growing interest.
Market Restraints
Ethical and Regulatory Concerns: Use of genetic information raises ethical issues, including data privacy and potential discrimination. Regulatory frameworks need to evolve to address these concerns and ensure responsible use of genomic tests.
Shortage of Skilled Professionals: The field of genomics requires specialized expertise for interpreting genetic data. The current shortage of qualified professionals may impede the widespread adoption of genomic diagnostics.
Reimbursement Challenges: Unclear reimbursement pathways within the National Health Service (NHS) can limit the integration of genomic tests into clinical practice, affecting their accessibility and utilization.
Regulatory Landscape and Reimbursement Scenario
In the UK, the regulatory landscape for genomic diagnostics involves several key bodies. The Medicines and Healthcare Products Regulatory Agency (MHRA) oversees in vitro diagnostics under current EU Medical Device Directives, with new UK Medical Device Regulations (MDR) set to apply by July 2024. The National Institute for Health and Care Excellence (NICE) evaluates the clinical and cost-effectiveness of genomic tests, influencing their adoption and reimbursement decisions. The transition to UK MDR introduces some uncertainty for manufacturers and developers.
Reimbursement for genomic tests primarily depends on their inclusion in the National Genomic Test Directory (NGTD), maintained by the National Health Service (NHS). NHS commissioning groups make regional decisions on which tests to fund, leading to variations in access across different areas. Challenges include aligning NICE's value assessments with the rapid evolution of genomic tests and clarifying data protection regulations related to genomic diagnostics.
Competitive Landscape
Key Players
Here are some of the major key players in the UK Genomic Diagnostics
- Illumina, Inc.
- Myriad Genetics, Inc.
- Amgen, Inc.
- 23andMe
- AncestryDNA
- Helix OpCo LLC
- F. Hoffmann-La Roche AG
- Abbott Laboratories
- Danaher Corporation
- Igenomix
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UK Genomic Diagnostics Market Segmentation
By Technology
- Next Generation Sequencing
- Array Technology
- PCR-based Testing
- FISH
- Others
By Application
- Ancestry & Ethnicity
- Traits Screening
- Genetic Disease Carrier Status
- New Baby Screening
- Health and Wellness-Predisposition/Risk/Tendency
By Product
- Consumables
- Equipment
- Software & Services
By End-user
- Hospitals & Clinics
- Diagnostic Laboratories
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































