UK ENT Devices Market Analysis
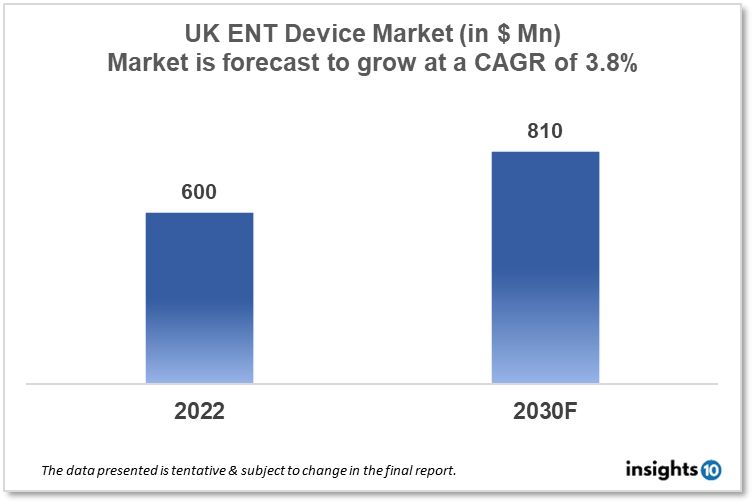
UK ENT Devices Market is projected to grow from $600 Mn in 2022 to $810 Mn by 2030, registering a CAGR of 3.8% during the forecast period of 2022-30. The rising prevalence of ENT disorders, such as hearing loss, sinusitis, and tonsillitis, is a major driver of the ENT device market. The market is highly competitive, with a large number of players operating in the space, ranging from small, specialized companies to large multinational corporations. Some of the key players in the UK ENT devices market include Otomagnetics, Keystone ENT, and AventaMed.
Buy Now

UK ENT Devices Market Analysis Summary
UK ENT Devices Market is projected to grow from $600 Mn in 2022 to $810 Mn by 2030, registering a CAGR of 3.8% during the forecast period of 2022-30.
The UK is a high-income, developed island country spanning an archipelago including Great Britain, located in Western Europe comprising England, Scotland, Wales, and Northern Ireland. In the United Kingdom, there are around 2,300 ENT healthcare professionals and medical practitioners.
ENT UK is the professional membership body in the UK that represents ear, nose, and throat surgery as well as head, neck, and thyroid surgery. Ménière's disease affects around one in every 1,000 people in the United Kingdom. UK's government spent 12 % of its GDP on healthcare in 2020.
Market Dynamics
Market Growth Drivers Analysis
Cochlear implants have been the most recent breakthrough, allowing the profoundly deaf to hear for the first time. The advancement of lens technology has allowed for unprecedented views within the nose and sinuses, as well as a far better understanding of their disorders. Most ENT surgical procedures, such as balloon sinus dilation and endoscopic sinus surgery, computer-assisted surgical navigation, tongue suspension and hyoid suspension for the treatment of obstructive sleep apnea (OSA), and inferior turbinoplasty procedures, are reimbursed in developed countries such as the UK. These aspects could boost UK ENT Devices Market.
Market restrains.
People's access to care for ear illnesses and hearing loss is frequently hampered by a lack of correct information and stigmatising attitudes toward these conditions. Healthcare cost reduction has emerged as a major focus area in healthcare systems during the previous decade. To accomplish this, price controls, competitive pricing, bidding, tender mechanics, coverage and payment policies, comparative effectiveness of therapies, technology assessments, and managed-care agreements are employed. These factors may deter new entrants into the UK ENT Devices Market.
Competitive Landscape
Key Players
- AventaMed - A company based in Galway, Ireland that produces a medical device for ear tube placement. They also have offices in the UK
- Keystone ENT - A company based in Lancashire, UK that produces a range of ENT instruments, including ear specula, forceps, and suction tubes
- PanMed UK - A company based in Kent, UK that produces a range of ENT instruments, including ear specula, forceps, and microscopes
- Endovision - A company based in West Sussex, UK that produces endoscopic equipment for use in ENT and other medical specialties
- Xion Medical - A company based in Surrey, UK that produces a range of ENT instruments, including nasal specula, forceps, and suction tubes.
- Otomagnetics - Based in Oxford, UK, this company produces a magnetic delivery system for medication to the ear
- Rhinix - Based in Copenhagen, Denmark, this company has a UK office and produces nasal dilators for the treatment of nasal obstruction and snoring
- Medicina - Based in Yorkshire, UK, this company produces a range of medical devices for use in ENT, including laryngoscopes, nasal splints, and ear syringes.
- Endoscope-i
- Kestrel Medical
Recent Notable Updates
February 2023: The 10th International Conference on Otolaryngology and ENT Surgery was held in February 2023 in London, UK, and comprised timely keynote presentations, oral talks, poster presentations, and exhibitions. The conference's theme was "Advancement of the most recent research in Otolaryngology and ENT Surgery.”
May 2022: The "3rd World Congress on Otolaryngology-Head & Neck Surgery" will be held in Edinburgh, Scotland, in May 2022. ENT 2022, which intended to bring together the most exquisite societies and enterprises, as well as known and honourable individuals from top colleges around the world. International exhibitions from corporate sectors were also welcomed to present the most recent breakthroughs in technologies and procedures.
Healthcare Policies and Reimbursement Scenarios
In the UK, the regulation and reimbursement of ENT (Ear, Nose, and Throat) devices are overseen by various bodies, including the Medicines and Healthcare Products Regulatory Agency (MHRA) and the National Institute for Health and Care Excellence (NICE). The MHRA is responsible for regulating medical devices in the UK. This includes ENT devices, such as hearing aids, cochlear implants, and nasal dilators. The reimbursement of ENT devices in the UK is managed by the National Health Service (NHS). NICE provides guidance on the use of medical technologies in the UK healthcare system, including ENT devices.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
ENT Device Market Segmentation
The ENT Device Market is segmented as mentioned below:
By Product Type (Revenue, USD Billion):
- Diagnostic Devices
- Surgical Devices
- Hearing Aids
- Hearing Implants
- Co2 Lasers
- Image-Guided Surgery Systems
By Diagnostic Devices (Revenue, USD Billion):
- Endocsopes
- Hearing Screening Devices
By Surgical Device (Revenue, USD Billion):
- Powered Surgical Instruments
- Radiofrequency (RF) Handpieces
- Handheld Instruments
- Balloon Sinus Dilation Devices
- ENT Supplies
- Ear Tubes
- Voice Prosthesis Devices
By End Users (Revenue, USD Billion):
- Hospitals and Ambulatory Settings
- Home Use
- ENT Clinics
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































