UK Dilated Cardiomyopathy Therapeutics Market Analysis
UK Dilated Cardiomyopathy Therapeutics Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. The phrase "dilated cardiomyopathy" refers to conditions that affect the heart muscle and is regarded as a deadly cardiac condition. Reduced myocardial contractility and left and right ventricular dilatation are usually its defining characteristics. The potential driving force behind the DCM Therapeutics Market has been the rise in diagnostic alterations and symptom awareness, as well as declining dietary and lifestyle choices that have led to an increase in the occurrence of disease. The government is sponsoring research and development (R&D) projects to encourage scientists and pharmaceutical firms to create novel treatments. Array BioPharma, Inc., AstraZeneca plc, Celladon Corporation, GlaxoSmithKline plc, Janssen Pharmaceuticals, Inc. (J&J), Merck & Co., Inc., Novartis International AG, Pfizer, Inc., Sanofi S.A., Teva Pharmaceutical Industries Ltd., Vericel Corporation, and many others are key players in the market for cystic fibrosis (CF) therapeutics.
Buy Now

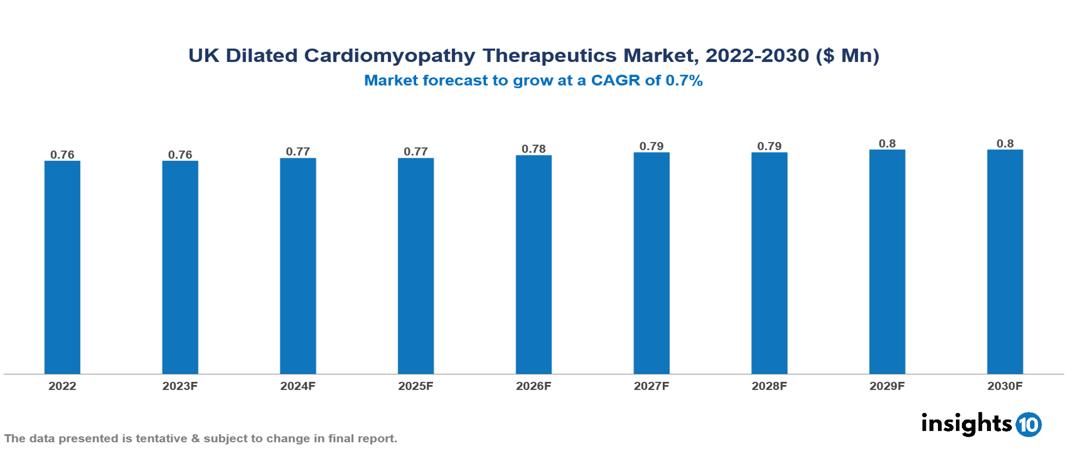
UK Dilated Cardiomyopathy Therapeutics Market Analysis Summary
UK Dilated Cardiomyopathy Therapeutics Market is valued at around $0.76 Mn in 2022 and is projected to reach $0.8 Mn by 2030, exhibiting a CAGR of 0.7% during the forecast period 2023-2030.
The term "dilated cardiomyopathy" (DCM) describes the myocardium's weakness, consequent dilatation, and malfunction. Concomitant hypertrophy is typically present as well. The heart is typically enlarged and flabby in DCM. In DCM, there are no distinct histologic abnormalities. DCM has a variety of causes, including genetic factors (30 to 50% of cases), toxic factors (such as alcohol and chemotherapy drug doxorubicin), metabolic factors (such as iron overload in hereditary hemochromatosis), viral factors, and antecedent myocardial infarction. Nearly 20% of instances of DCM are caused by mutations in the TTN gene, which codes for the Titin protein. Dilated cardiomyopathy is frequently brought on by the Trypanosome cruzi-caused Chagas disease in Latin America.
The potential driving force behind the DCM Therapeutics Market has been the rise in diagnostic alterations and symptom awareness, as well as declining dietary and lifestyle choices that have led to an increase in the occurrence of disease. The government is sponsoring research and development (R&D) activities in an effort to encourage scientists and pharmaceutical businesses to create novel medications.
Array BioPharma, Inc., AstraZeneca plc, Celladon Corporation, GlaxoSmithKline plc, Janssen Pharmaceuticals, Inc. (J&J), Merck & Co., Inc., Novartis International AG, Pfizer, Inc., Sanofi S.A., Teva Pharmaceutical Industries Ltd., Vericel Corporation, and many others are key players in the market for cystic fibrosis (CF) therapeutics.
Market dynamics
Market Drivers
Different diagnostic tests used to treat peripartum cardiomyopathy are increasing, which is helping the market's growth. To find the presence of particular abnormalities, various techniques are used, such as cardiac MRI and electrocardiography. As a result, it plays a key role in the market's expansion.
Increasing The market for DCM therapies is also being driven by the prevalence of DCM.
Changes in Lifestyle: Smoking is one of the top causes of preventable illness and death in the United States. Every year, it causes more than 480,000 deaths. Additionally, the modern population's adopted lifestyles that include an unhealthy way of living, a poor diet, stress, and many other similar problems all contribute to the market's expanding prospects.
An increase in research and development (R&D) activities: R&D activities are one factor boosting the market's growth. As a result, the market for dilated cardiomyopathy will benefit globally. The government is sponsoring research and development (R&D) projects to encourage scientists and pharmaceutical firms to create novel treatments. For instance, TN-301 (TYA-11631) is being developed to treat hereditary dilated cardiomyopathy and heart failure (HF) with preserved ejection fraction (HFpEF) (DCM). Oral administration is the method used. The potential medication works by inhibiting histone deacetylase 6. (HDAC6).
Development in Dilated Cardiomyopathy Therapeutics Market
For the treatment of hereditary dilated cardiomyopathy and heart failure (HF) with preserved ejection fraction (HFpEF), TN-301 (TYA-11631) is being developed (gDCM). Oral administration is the method used. The potential medication works by inhibiting histone deacetylase 6. (HDAC6).
In order to meet important clinical requirements for people with hereditary heart disease, MyoKardia is creating a pipeline of innovative small-molecule medicines. One million persons in the United States suffer from hypertrophic and dilated cardiomyopathy combined, and for which no innovative treatments have been made available in the market in more than ten years.
Preclinical AAV-based gene therapy programmes are being developed by Rocket Pharmaceuticals for the treatment of PKP2-arrhythmogenic cardiomyopathy (ACM) and BAG3-associated dilated cardiomyopathy (DCM).
Key players
Novartis Pfizer Johnson & Johnson Amgen Celgene UCB Takeda Pharmaceutical Company Vertex Pharmaceuticals Gilead Sciences Sanofi1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market segmentations for UK Dilated Cardiomyopathy Therapeutics Market
By Drug class
- Angiotensin-converting enzyme (ACE) Inhibitors
- Beta-blockers
- Aldosterone antagonists,
- Angiotensin II Receptor Blockers
- Others
By Devices
- Implantable Devices
- Heart Pumps
- Cardioverter-Defibrillators
- Others
By Route of Administration
- Oral
- Parenteral
- Others
By End-User
- Hospitals
- Research Institutes
- Specialty Clinics
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.










































