UK Clinical Trial Supply & Logistics for Pharmaceutical Market Analysis
UK Clinical Trial Supply & Logistics for Pharmaceuticals Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. The market for Clinical Trial Supply & Logistics for Pharmaceuticals Market is growing due to rapid increase in clinical trials, implementation of strict regulations, increase in research and development expenditure by pharmaceutical and biopharmaceutical firms, and advancements in technology. Some of the key players in the global Clinical Trial Supply & Logistics for Pharmaceuticals Market include Thermo Fisher Scientific (Patheon), Catalent, Inc., Parexel, Almac Group, Marken, Piramal Pharma Solutions, UDG Healthcare, DHL, FedEx, Movianto, and Packaging Coordinators Inc.
Buy Now

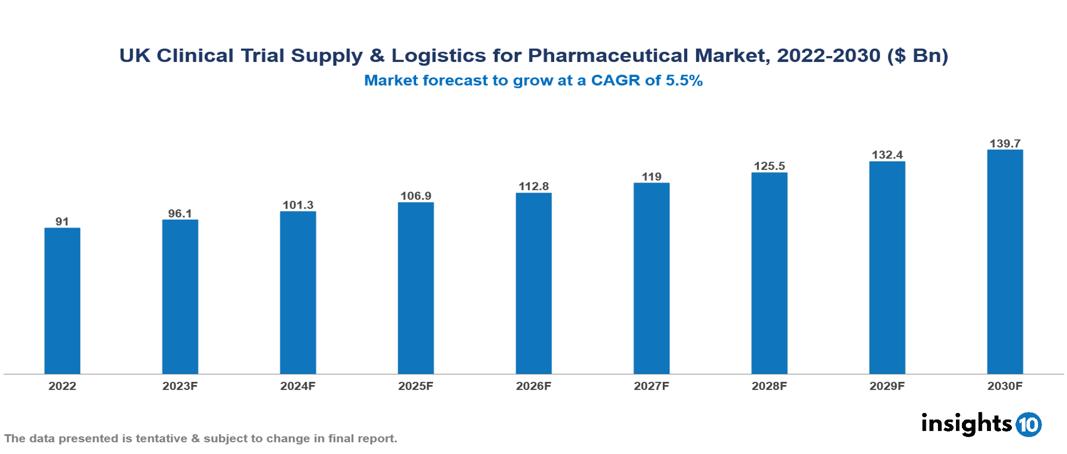
UK Clinical Trial Supply & Logistics for Pharmaceutical Market Executive Summary
UK Clinical Trial Supply & Logistics for Pharmaceutical Market is valued at around $91 Bn in 2022 and is projected to reach $139.7 Bn by 2030, exhibiting a CAGR of 5.5% during the forecast period 2023-2030.
Clinical trial supply & logistics entails the management of clinical supplies. These are required for clinical investigations of pharmaceuticals or medical devices as per convention and appropriate regulatory standards. This procedure includes overseeing the planning, packaging, labelling, forecasting, sourcing, and distribution of clinical goods for business and government sponsors whose clinical trials are in phases 1-4.
A significant investment in supply chain management software by clinical trial supplies providers has been seen. The adoption of technologies in new supply chain management is due to the rise in complexities in clinical trials and increased competition among market players.
Clinical trial providers coordinate with many third-party vendors and technicians during different stages of clinical distribution to confirm whether the study drugs are accessible in sufficient amounts and quantity.
Pharmaceutical and biotechnology firms like Thermo Fisher Scientific (Patheon), Catalent, Inc., Parexel, Almac Group, Marken, Piramal Pharma Solutions, UDG Healthcare, DHL, FedEx, Movianto, and Packaging Coordinators Inc., are some of the major players in the Clinical Trial Supply & Logistics for Pharmaceuticals Market.
The factors accountable for driving the clinical trial logistics market around the world are the substantial developments in the field of healthcare and medication, in terms of testing, improvement of new operations, investigations, and medications.
Market Dynamics
Drivers of UK Clinical Trial Supply & Logistics for Pharmaceuticals Market:
Growing Incidence and Prevalence of Chronic Diseases: The market for clinical trial logistics is expanding with the rise in chronic diseases like cardiovascular diseases, cancer, obesity, and diabetes. These diseases will call for clinical trials for drug development which in turn will demand more clinical trial supply & logistics.
Increasing R&D Expenditure: Companies are investing in research to discover new drugs and treatments to meet the medical needs of the people. This leads to an extensive increase in the clinical trial supply & logistics pharmaceuticals market.
Increasing Clinical Trials: Clinical trials have been increasing globally leading to efficient supply chain management, which, in turn, is giving rise to clinical trial supply & logistics.
Increased Focus on Drug Development: The rise in chronic diseases has led pharmaceutical companies to invest in drug development to combat these diseases. This requires more clinical trials to be conducted leading to an increase in clinical trial supply & logistics for the pharmaceuticals market.
Restraints of UK Clinical Trial Supply & Logistics for Pharmaceuticals Market:
Cost Implications: Conducting clinical trials can be expensive as it can be a long process requiring costs for patient management, equipment, and overall execution.
Notable Deals in Clinical Trial Supply & Logistics for Pharmaceuticals Market:
In 2022, GlaxoSmithKline, a pharmaceutical and biotechnology company, acquired Sierra Oncology, a biopharmaceutical company focusing on targeted therapies for the treatment of rare forms of cancer, for $1.9 billion.
In 2022, Biohaven Pharma, a biopharmaceutical company, acquired Channel Biosciences, a non-government company proving process engineering solutions to biotechnology and related industries, for $1.2 billion.
In 2022, AbbVie, a pharmaceutical company, acquired Syndesi Therapeutics, a biotechnology company specializing in neurology drug discovery to develop potential therapeutics in cognitive disorders, for $1 billion.
In 2022, Halozyme Therapeutics, a biotechnology company, acquired Antares Pharma, a pharmaceutical company that combines drug development expertise with proprietary delivery technology to deliver safe and effective medicines, for $0.9 billion.
Key players
Almac Group Limited Catalent, Inc. DHL International GmbH World Courier Management, Inc. PCI Pharma Services Parexel International Corporation Sharp Clinical Services Thermo Fisher Scientific Inc. Movianto UK Fisher Clinical Services, Inc.1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For UK Clinical Trial Supply & Logistics for Pharmaceuticals Market
By Service:
- Logistics & Distribution
- Storage & Retention
- Packaging, Labeling, and Blinding
- Manufacturing
- Comparator Sourcing
- Other Services
By Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
By End-user:
- Pharmaceuticals
- Biologicals
- Medical Device
By Therapeutic Area:
- Cardiovascular Diseases
- Respiratory Diseases
- CNS & Mental Disorders
- Oncology
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































