UK Biosensors Market Analysis
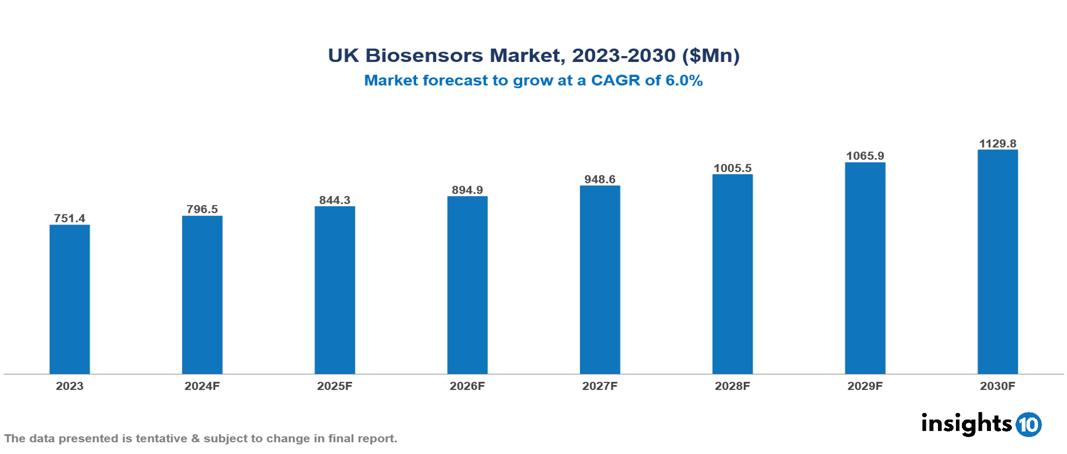
The UK Biosensors Market was valued at $751.4 Mn in 2023 and is predicted to grow at a CAGR of 6% from 2023 to 2030, to $1129.8 Mn by 2030. The key drivers of the market include increasing burden of chronic diseases, emphasis on preventative care, and technological advancements. The prominent players of the UK Biosensors Market are Lifesensors, Siemens Healthcare, B. Braun Melsungen AG, Meridian Bioscience, and Biosensors International, among others.
Buy Now

UK Biosensors Market Executive Summary
The UK Biosensors market is at around $751.4 Mn in 2023 and is projected to reach $1129.8 Mn in 2030, exhibiting a CAGR of 6.0% during the forecast period.
Biosensors are analytical devices that detect changes in biological processes and transform the biological data into electrical signal. On the basis of sensor device and biological material, biosensors are classified into many types including electrochemical, thermal, optical, piezo-electric biosensors, and many more variants of these. Electrochemical biosensors work on the principle that many enzyme-catalyzed reactions consume or generate ions or electrons which causes change in electrical properties of the analyte being detected and can be used as a measuring parameter. Electrochemical biosensors are further subdivided into amperometric, potentiometric, impedimetric, and voltametric biosensors. One of the main applications of biosensors in early diagnosis and prompt disease treatment
Given the prevalence of the four most common chronic diseases which include allergy, high blood pressure, low back disorder, and depression, there is a significant burden on the healthcare sector in the UK. The UK Biosensors Market is thus driven by significant factors such as the increasing burden of chronic diseases, emphasis on preventative care, and technological advancements. However, stringent regulatory requirements, technical challenges, and data privacy and security issues restrict the growth and potential of the market.
The major players of the UK Biosensors Market are Lifesensors, Siemens Healthcare, B. Braun Melsungen AG, Meridian Bioscience, and Biosensors International, among others.
Market Dynamics
Market Growth Drivers
Increasing Burden of Chronic Diseases: Allergy is the most common chronic health condition in both men and women with a prevalence rate of 30.4% and 36%, respectively. Certain environmental allergens, such as pollen, dust mites, pet dander, and food allergies, can be identified by biosensors. By using biosensors, people may be able to prevent their exposure to allergens that worsen their symptoms. Also, wearable biosensors have the ability to track physiological indicators of allergic reactions, including variations in skin temperature, heart rate, and breathing patterns. Users may receive early warning indicators of an allergic reaction from this real-time data. Thus, the effective allergy management through biosensors results in the growth of the Biosensors Market.
Emphasis on Preventative Care: The increasing prevalence of chronic illnesses is making preventative care more important in healthcare. Biosensors are crucial in this regard, as they help individuals monitor their health and detect early risk factors for chronic conditions. This early detection allows for preventative actions, like lifestyle changes or medications, which can delay the onset of chronic diseases. Thus, the growing emphasis on preventative care is fuelling the growth of the biosensors market.
Technological Advancements: Technological innovations in biosensors are progressing rapidly. The novel application of fluorescence tagging and nanomaterials such as graphene and carbon nanotubes has significantly increased biosensor sensitivity and detection limits. Advances in nanotechnology, materials science, and microfabrication have enabled the creation of smaller biosensors, which are more effective due to their enhanced sensitivity, specificity, and faster reaction times. Wearable biosensors now feature biocompatible materials to minimize the risk of rejection by the body and to ensure patient comfort and compliance. The integration of biosensors with AI and ML algorithms has improved data analysis accuracy and effectiveness, allowing for personalized healthcare strategies and early disease detection.
Market Restraints
Stringent Regulatory Requirements: The health benefits of biosensors, especially for medical use, are substantial. Companies must comply with GDPR and HIPAA regulations. Regulatory bodies rigorously test medical devices for accuracy, safety, and risks such as data security and biocompatibility. While strict guidelines protect patients and foster market trust, the lengthy and costly approval process can delay the launch of innovative biosensors. This can stifle innovation and limit patient access. Additionally, the high costs of regulatory compliance increase biosensor prices, hindering market expansion.
Technical Challenges: Reliable and reproducible results depend on long-term stability and shelf-life, which are influenced by factors like temperature fluctuations, enzyme degradation, and calibration drift. Developing smaller versions for wearable biosensors and point-of-care diagnostics is essential, but integrating them into the current healthcare infrastructure is challenging and requires further development. These technical challenges are obstacles to the growth of the Biosensors Market.
Data Management Challenges: Containing highly sensitive information such as patients' genetic data and health metrics, biosensor data needs to be safeguarded against misuse, unauthorized access, and breaches. As biosensors become more interconnected, they face increased vulnerability to cyberattacks, including data manipulation, operational disruption, and theft of sensitive information. Encryption is necessary to prevent unauthorized access, requiring the implementation of robust and costly authentication and access control measures. These factors significantly impact the biosensors market.
Regulatory Landscape and Reimbursement Scenario
The Medicines and Healthcare products Regulatory Agency (MHRA) is the government authority of UK, sponsored by the Department of Health and Social Care. MHRA ensures that pharmaceuticals, blood components, advanced therapy medical products, and medical devices meet applicable standards of safety, quality, and efficacy. Its core responsibilities include overseeing UK notified bodies, regulating clinical trials, monitoring compliance for medicines and medical devices, and offering technical and regulatory advice for these products. Moreover, MHRA serves to protect the overall public health by promoting international standardization and harmonization.
The license or marketing authorization for new drugs is granted by the regulatory authority, i.e., MHRA after ensuring that the drug is safe, effective, and meets GMP requirements. The MHRA is responsible for granting license in England, Scotland, and Wales and the European Medicines Agency (EMA) is responsible in Northern Ireland. Once the drug is granted a license, appraisal takes place before it is made available on the National Health Service (NHS). In England and Wales, this appraisal is carried out by the National Institute for Health and Care Excellence (NICE), in Scotland by Scottish Medicines Consortium (SMC), and in Northern Ireland by Department of Health (DoH).
The goal of the National Health Service (NHS), the UK’s tax-funded healthcare system, is to provide comprehensive healthcare services free at the point of access for its residents. Two primary methods of reimbursement exist: Primary Care and Secondary Care. Local Clinical Commissioning Groups (CCGs) are tasked for contracting with and compensating general practices for primary care services, such as doctor visits. For Secondary Care, NHS England oversees the funding of hospitals and specialized services centrally. Hospital reimbursement is determined by a national tariff system that establishes rates for particular medical services.
Competitive Landscape
Key Players
Here are some of the major key players in the UK Biosensors Market:
- Lifesensors
- Siemens Healthcare
- B. Braun Melsungen AG
- Meridian Bioscience
- Biosensors International
- Nix Biosensors
- Abbott Laboratories
- Medtronic
- Siemens Healthcare
- F. Hoffmann-La Roche
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UK Biosensors Market Segmentation
By Technology
- Electrochemical Biosensors
- Optical Biosensors
- Piezoelectric Biosensors
- Thermal Biosensors
- Nanomechanical Biosensors
By Product
- Wearable Biosensors
- Non-wearable Biosensors
By Application
- Medical Diagnostics
- Food Safety
- Environmental Monitoring
- Agriculture and Bioreactor Monitoring
- Other
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































