UK Anemia Therapeutics Market Analysis
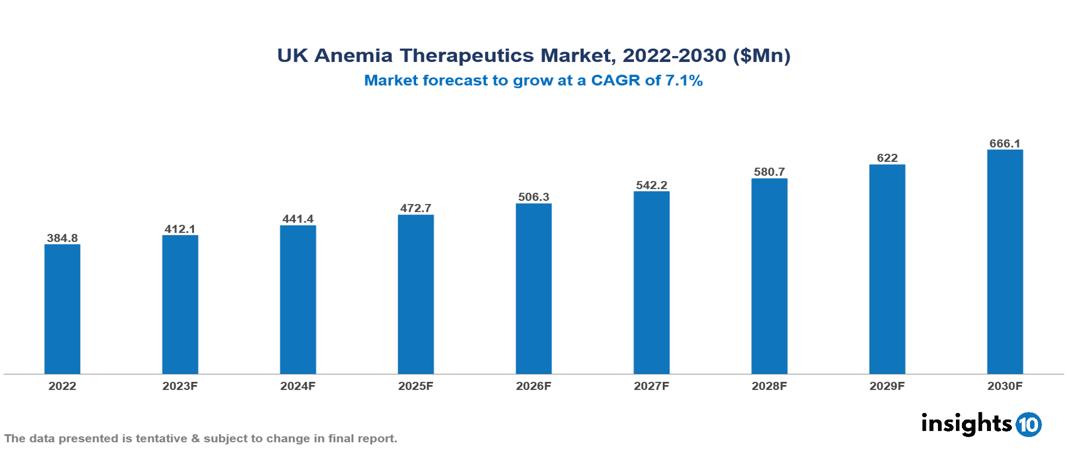
The UK Anemia Therapeutics Market is anticipated to experience growth from $385 Mn in 2022 to $666 Mn by 2030, with a CAGR of 7.1% during the forecast period of 2022-2030. The UK Anemia Therapeutics Market is driven by a number of factors, including an aging population with a rising prevalence of chronic illnesses, especially in pregnant women, the National Health Service's emphasis on affordable and easily accessible treatments, and continuous technological advancements. The UK Anemia Therapeutics Market encompasses various players across different segments, including Pfizer, Roche, GlaxoSmithKline (GSK), Novartis, Johnson & Johnson, Shield Therapeutics, Advanz Pharmaceutical, Hikma Pharmaceuticals, Amgen, Vifor Pharma, etc, among various others.
Buy Now

UK Anemia Therapeutics Market Analysis Executive Summary
The UK Anemia Therapeutics Market is anticipated to experience a growth from $385 Mn in 2022 to $666 Mn by 2030, with a CAGR of 7.1% during the forecast period of 2022-2030.
Anemia is a medical disorder characterized by a low concentration of hemoglobin in the blood or a shortage of red blood cells. This causes the blood's ability to carry oxygen less effectively, which causes symptoms including weakness, exhaustion, pale complexion, and shortness of breath. There are several kinds of anemia, each with unique causes and therapies. The most prevalent kind of anemia, iron deficiency, is caused by inadequate iron consumption or problems with iron absorption. Treatment options include iron supplements and dietary changes. Vitamin-deficiency anemias, including folate and B12 deficiencies, are caused by insufficient consumption of these vitamins, which calls for dietary modifications and treatment. Red blood cell breakdown occurs more quickly in hemolytic anemias, which calls for treating the underlying cause and, in extreme situations, blood transfusions. Aplastic anemia, which is defined by decreased production of red blood cells as a result of damage to the bone marrow, necessitates extensive care that may involve bone marrow transplantation in certain cases as well as treating the underlying cause. For symptoms to be successfully relieved and blood function to be restored, therapy for anemia must be customized to the particular kind and cause of the condition.
Anemia has been estimated to affect about 23% of pregnant women in the UK on a year-round basis. Approximately 12% of non-pregnant women between the ages of 15 and 49 have anemia. The frequency for kids between the ages of 6 and 59 months is about 5%. The UK Anemia Therapeutics Market is driven by a number of factors, including an aging population with a rising prevalence of chronic illnesses, especially in pregnant women, the National Health Service's emphasis on affordable and easily accessible treatments, and continuous technological advancements.
Leading pharmaceutical company, Pfizer offers well-known medications for a number of anemia forms, such as aplastic anemia (Revatio), iron insufficiency (Hemacrit), and vitamin B12 deficiency (Cobalamin). Amgen, a somewhat smaller firm, treats anemia linked to secondary hyperparathyroidism and chronic renal disease, respectively, with Sensipar and Epogen. Companies based in the UK, such as Advanz Pharmaceuticals and Hikma Pharmaceuticals, are popular among the population there and provide generic versions of several medications.
Market Dynamics
Market Growth Drivers
Demographic Dynamics: One important element is the aging population in the UK. Chronic illnesses like cancer and renal disease, which frequently result in anemia, are becoming more common as people live longer. This results in a greater number of patients who need to have their anemia managed, which increases market demand. In the UK, anemia affects about 23% of expectant mothers. This susceptible population drives market trends toward safe and efficacious treatments for maternal health. It also requires specialized treatment choices and increased knowledge of potential consequences.
Encouraging Medical Environment: The National Health Service (NHS) places a high priority on giving patients access to reasonably priced and efficient anemic treatments. This promise guarantees steady demand for well-proven treatments and creates opportunities for the uptake of fresh, research-proven solutions. Government initiatives to combat iron deficiency and enhance maternal health also support market growth. These programs encourage preventive care, increase public knowledge, and obliquely aid in the creation and use of successful treatments.
Innovations in Technology: Specialized market sectors are seeing expansion due to ongoing research on gene therapy, stem cell transplantation, and new therapeutic targets for uncommon and treatment-resistant types of anemia. The treatment of anemia may be much improved in the future with the help of these novel medicines. Long-acting injectable iron sucrose is one example of a medication delivery system advancement that improves patient compliance and convenience. This encourages market adoption by enhancing treatment adherence and perhaps lowering healthcare expenses.
Market Restraints
Budgetary concerns: The NHS prioritizes treatments that are most economical despite operating under strict budgetary restrictions. This may make it more difficult for newer, costlier anemia treatments to be adopted, even if they provide better convenience or efficacy. Policies regarding drug reimbursement, particularly for more recent and focused treatments, may be restrictive. Patients face major obstacles as a result, and promising medications have less opportunity to enter the market.
Difficult Competition: Market share for more recent, branded formulations can be greatly impacted by the availability of well-known, less expensive generic copies of earlier medications. This may impede the market's expansion for novel treatments unless they provide clearly better clinical outcomes or ease of use. Indirectly impacting certain market sectors, the focus of public health campaigns on balanced meals and iron supplements as prophylactic measures against iron deficiency anemia may result in a smaller market for treatment pharmaceuticals.
Strict Regulations: Strict guidelines are followed by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) before approving drugs. Stricter regulations protect patient safety, but they can also cause long delays in new drug launches, delaying patient access to potentially life-saving therapies. Incentives for pharmaceutical companies to invest in research and development of novel therapies tailored to specific patient populations may not always be sufficiently provided by regulatory frameworks, which could impede the advancement of treating uncommon or complicated forms of anemia.
Healthcare Policies and Regulatory Landscape
The UK's dedication to provide its residents affordable, first-rate healthcare has influenced the country's healthcare policy. A major player is the National Health Service (NHS), which provides extensive and mostly free healthcare services. Preventive care, public health campaigns, and fair access to medical care are the main focuses of healthcare policy. In order to maintain these regulations, the Medicines and Healthcare Products Regulatory Agency (MHRA) is an essential organization. The MHRA is the UK's regulatory body that protects the quality, safety, and efficacy of pharmaceuticals, medical equipment, and blood components. It evaluates and approves their use, keeps an eye on their safety after approval, and adds to the healthcare system as a whole by protecting public health via strict control and monitoring. In line with the more general objectives of UK healthcare policy, the MHRA's function is essential to preserving the efficacy and integrity of the healthcare system.
Competitive Landscape
Key Players:
- Pfizer
- Roche
- GlaxoSmithKline (GSK)
- Novartis
- Johnson & Johnson
- Shield Therapeutics
- Advanz Pharmaceutical
- Hikma Pharmaceuticals
- Amgen
- Vifor Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UK Anemia Therapeutics Market Segmentation
By Type of Disease
- Iron Deficiency Anemia
- Megaloblastic Anemia
- Pernicious Anemia
- Hemorrhagic Anemia
- Hemolytic Anemia
- Sickle Cell Anemia
By Population
- Pediatrics
- Adults
- Geriatrics
By Therapy Type
- Oral Iron Therapy
- Parenteral Iron Therapy
- Red Blood Cell Transplantation
- Others
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Online Pharmacies
By End User
- In-Patient Centres
- Out-Patient Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.