UAE Pharmacovigilance Market Analysis
UAE Pharmacovigilance Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Pharmacovigilance is expanding as a result of increasing demand of new and safer drugs. The demand for pharmacovigilance services is fueling due to the regulatory mandates on clinical trial conduct and the post-marketing vigilance. Some of the key players in the global Pharmacovigilance Market include Cognizant, Capgemini, Accenture, Wipro Limited, IBM, IQVIA, Laboratory Corporation of American Holdings, Clinquest Group B.V., ICON Plc., PAREXEL International Corporation, United Biosource Corporation, and Take Solutions.
Buy Now

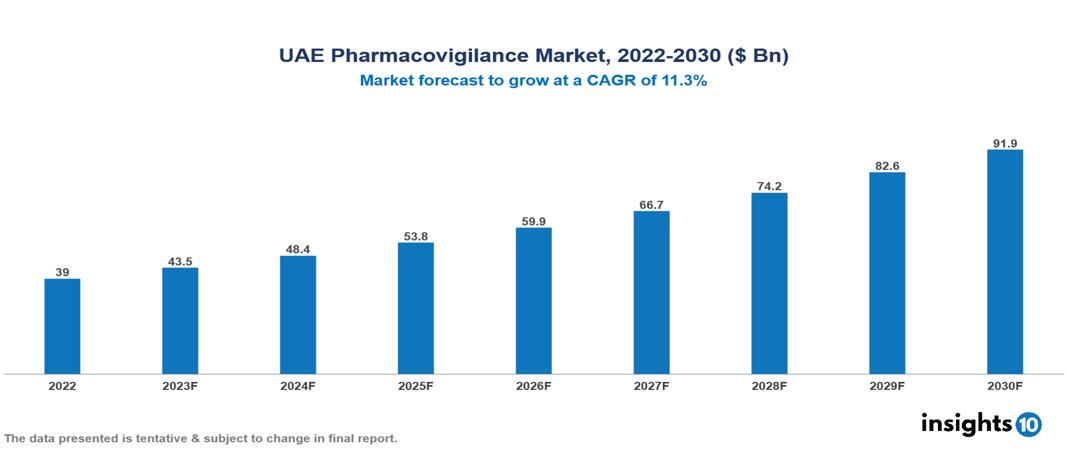
UAE Pharmacovigilance Market Executive Summary
UAE Pharmacovigilance Market is valued at around $39 Bn in 2022 and is projected to reach $91.8 Bn by 2030, exhibiting a CAGR of 11.3% during the forecast period 2023-2030.
Worldwide drug consumption has increased as a result of a rise in the prevalence of chronic diseases like oncological diseases, diabetes, and cardiovascular and respiratory problems. As a result, there is a greater need for developing new medications through extensive clinical studies. Pharmacovigilance (PV) is a necessary step in the process of finding new drugs and developing them. The demand for Pharmacovigilance services is anticipated to increase as adverse drug reactions (ADRs) become more common.
Post-marketing medication review is critical in order to fill the data gap left by pre-marketing research and to better characterize drug safety profiles in real-world contexts. Such research will emphasize the value of pharmacovigilance even more, leading to market expansion.
Organizations like Cognizant, Capgemini, Accenture, Wipro Limited, IBM, IQVIA, Laboratory Corporation of American Holdings, Clinquest Group B.V., ICON Plc., PAREXEL International Corporation, United Biosource Corporation, and Take Solutions, are some of the major players in the Pharmacovigilance Market.
Pharmacovigilance service outsourcing is one of the key trends fueling the sector's expansion. This is because of outsourcing's various benefits, which include scalability, cost-effectiveness, access to knowledge, regulatory compliance, and cost efficiency. Pharmacovigilance services contract outsourcing is widely used among pharmaceutical companies since it is an economically feasible way to meet drug safety requirements while putting a strong emphasis on core business operations.
Another important element boosting market growth is the rise in the frequency of adverse medication responses. Due to the significant level of patient variability in pharmacokinetics and pharmacodynamics, the majority of adverse drug reactions are caused by the prolongation of a drug's intended pharmacologic effects.
Market Dynamics
Drivers of UAE Pharmacovigilance Market:
Increasing Incidence of Life-style Related Diseases: As a result of sedentary lifestyles, a lack of physical activity, evolving lifestyle patterns, and poor diets, lifestyle-related diseases like diabetes, hypertension, and cardiac disorders are on the rise. Increased drug consumption due to these diseases indicates a high demand for drug monitoring and is anticipated to drive the growth of the Pharmacovigilance market.
Increasing Public Awareness for Safer Drugs: Patients are becoming more aware of which drugs are safer to use, driving the growth of the Pharmacovigilance market.
Government Regulatory Measures: The need for pharmacovigilance solutions is growing due to strict regulatory guidelines governing drug distribution and an increase in the number of adverse events brought on by medications. This is creating new potential for the pharmacovigilance market to expand.
Increasing Trend of Outsourcing Pharmacovigilance Services: Outsourcing offers benefits with respect to time constraints and facilities and allows the companies to undergo customization as per their requirements, increasing the speed of the evaluation process, hence, propelling the growth of the Pharmacovigilance Market.
Notable Deals in Pharmacovigilance Market:
In 2022, Cognizant entered into a partnership with Medable Inc. to jointly deliver clinical research solutions based on Medable’s software-as-a-service platform for decentralized clinical trials.
In 2022, LINK Medical and Viedoc entered into a partnership established by Viedoc and designed to improve trial efficiency for LINK Medical and its clients.
Key players
Clinfound Clinical Research LLC 3D Communication Pharmacovigilance Consulting Services (PVCS) ProPharma Group RAS Pharma & Logistics Services MakroCare Emedcare PhaRA Regulatory Affairs Quality Regulatory Consultants (QRC) PrimeVigilance Ltd.1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For UAE Pharmacovigilance Market
By Product Life Cycle
- Pre-Clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Third Parties
By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
By Process Flow
- Case Data Management
- Signal Detection
- Risk Management System
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Pulmonology
By End-user
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































