UAE Cytomegalovirus Therapeutics Market Analysis
UAE Cytomegalovirus Therapeutics market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. A member of the Herpesviridae family with an envelope, the human herpesvirus 5 (HHV-5) is also referred to as cytomegalovirus. Around 40% to 100% of adults worldwide have experienced HHV-5 exposure at some point in their lifetime, making it a very common virus. A rise in the frequency of cytomegalovirus infection, investments in research & development, and the introduction of novel medicines are some of the reasons driving the growth of the market for cytomegalovirus therapeutics. Several Global pharmaceutical firms, including Moderna, China Immunotech, Nobelpharma, Hookipa Biotech GmbH, Biotest, AlloVir, Merck Sharp & Dohme LLC, Chimerix, VBI Vaccines, Atara Biotherapeutics, SpyBiotech, MEMO Therapeutics, Lion TCR, and others are active in this Cytomegalovirus Therapeutics market.
Buy Now

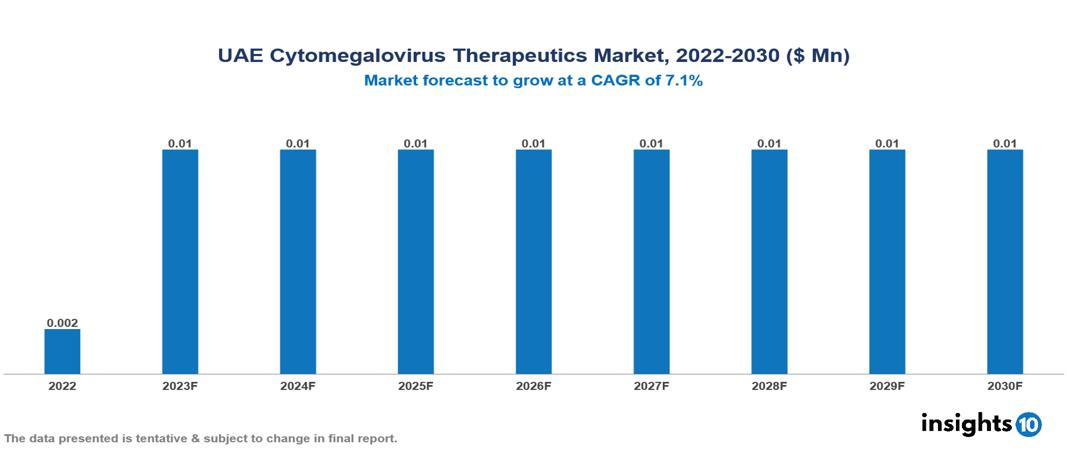
UAE Cytomegalovirus Therapeutics Market Analysis Summary
UAE Cytomegalovirus Therapeutics Market is valued at around $0.002 Mn in 2022 and is projected to reach $0.01 Mn by 2030, exhibiting a CAGR of 7.1% during the forecast period 2023-2030.
The widespread virus cytomegalovirus (CMV) can cause severe end-organ dysfunction in immunocompromised patients with congenital CMV illness, or it might be asymptomatic in healthy individuals. The herpesvirus family, Herpesviridae, or human herpesvirus-5, includes the human cytomegalovirus (HHV-5). Salivary glands and human cytomegalovirus infections are frequently linked. CMV infections may not cause any symptoms in healthy individuals, but they can be fatal in immunocompromised patients. Immunocompromised patients can develop a wide range of clinical illnesses from CMV, such as febrile syndromes, hepatitis, pneumonitis, retinitis, encephalopathy, esophagitis, and colitis.
The rise in the frequency of cytomegalovirus infection, investments in research & development, and the introduction of novel medicines are the main drivers of market growth for cytomegalovirus infection. In addition to Moderna, China Immunotech, Nobelpharma, Hookipa Biotech GmbH, Biotest, AlloVir, Merck Sharp & Dohme LLC, Chimerix, VBI Vaccines, Atara Biotherapeutics, SpyBiotech, MEMO Therapeutics, Lion TCR, and others, there are other significant in Cytomegalovirus Therapeutics market.
Market dynamics
Market Development
According to Takeda, Livtencity, or maribavir, has been approved by the FDA for use in patients 12 years of age and older who have post-transplant cytomegalovirus (CMV) infection and are resistant to one of four available medications. Takeda estimates that out of the 200,000 adult transplants performed each year around the world, roughly 25% of transplant recipients may get CMV infections. A peak sales estimate of $700 million to $800 million for Livtencity has been made by the Japanese pharmaceutical company using those numbers.
A leading Phase III programme for Cytomegalovirus (HHV-5) Infections has been developed for MRNA-1647, an M-RNA vaccine sold by Moderna. Preventing Cytomegalovirus (CMV) Disease in High-Risk Adult Kidney Transplant Recipients is a New Indication for PREVYMIS® (letermovir), a drug manufactured by Merck.
Market Drivers
Rise in the prevalence of cytomegalovirus infection
The market's growth rate between 2022 and 2030 is mostly driven by the rising prevalence of CMV infection. According to the Centers for Disease Control and Prevention, one in every 150 infants in the US is born with congenital cytomegalovirus (CMV) infection. Only one in five newborns who develop CMV go on to have long-term health problems. Due to increased infection rates and demand for better treatment options, the market for CMV infection treatment and diagnosis will grow.
Growing prevalence of viral infections
As AIDS patients' immune systems deteriorate, the prevalence of viral infections among them rises, increasing the requirement for anti-cytomegalovirus medications. In 2017, the CDC reported that 36.7 million persons worldwide were infected with the human immunodeficiency virus (HIV). It is anticipated that the availability of antiviral drugs to treat this ailment would increase demand for efficient treatment alternatives. This will affect how quickly the market expands.
Increase in the number of research and development activities
In the upcoming years, it is predicted that the growing number of research and development initiatives for novel indications and the patent expiration of biologics would present new chances for the expansion of the cytomegalovirus market. Major institutes have started a number of projects centred on R&D initiatives related to CMV infection. For instance, in August 2019, the University of Washington's Program for Advanced Cell Therapy (PACT) began a pioneering cell-therapy trial for kidney transplant patients, using virus-specific white blood cells to treat patients with severe cytomegalovirus (CMV) infection following kidney transplantation.
Other drivers
Furthermore, the market for CMV will grow because of people's sedentary lifestyles and the increasing number of government campaigns to raise awareness. Along with this, growing healthcare costs and patent expirations that result in the availability of generic pharmaceuticals will accelerate the market's growth pace. Additionally, it is predicted that an increase in organ transplantation around the globe will accelerate market growth.
Market Restraints
The market for CMV treatments is being hampered by falling transplant rates and declining CMV prevalence brought on by highly active antiretroviral therapy (HAART). Cryotherapy and laser therapy are a couple of the alternatives that compete for market share.
The high price of the treatment, on the other hand, will limit the market's ability to expand. The cytomegalovirus market will face obstacles from a lack of healthcare facilities in emerging nations and a shortage of qualified workers. During the projection period of 2022–2030, lack of public awareness and drug side effects would also act as a restraint and limit market expansion.
This cytomegalovirus industry research contains information on the latest trends, trade laws, import-export analysis, production analysis, value chain optimization, market share, and the effects of domestic and international factors.
Key players
Gilead Sciences AbbVie Merck & Co. Bristol-Myers Squibb Roche Novartis Pfizer Sanofi AstraZeneca Takeda1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UAE Cytomegalovirus Therapeutics Market Segmentation
By Diagnosis
- Serologic Test
- Polymerase Chain Reaction (PCR) Test
- Others
By Diseases
- Retinitis
- Pneumonia
- Gastrointestinal Ulcers
- Encephalitis
- Others
By Drug
- Cidofovir
- Foscarnet
- Valganciclovir
- Ganciclovir
- Others
By Route of Administration
- Oral
- Parenteral
- Others
By Application
- Stem Cell Transplantation
- Organ Transplantation
- Congenital CMV Infection
- Other
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































