UAE Cardiac Pacemakers Market Analysis
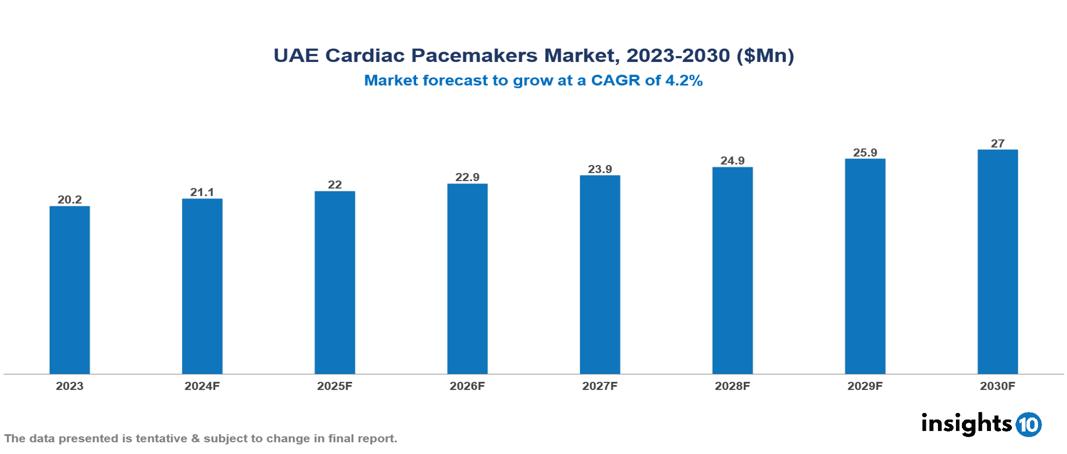
The UAE Cardiac Pacemakers Market was valued at $20.23 Mn in 2023 and is predicted to grow at a CAGR of 4.2% from 2023 to 2030, to $26.99 Mn by 2030. The key drivers of this industry include the high prevalence of cardiovascular diseases, advancements in technology, and government support. The industry is primarily dominated by players such as Abbott Laboratories, Biotronik, Boston Scientific, and Medtronic among others.
Buy Now

UAE Cardiac Pacemakers Market Executive Summary
The UAE Cardiac Pacemakers Market was valued at $20.23 Mn in 2023 and is predicted to grow at a CAGR of 4.2% from 2023 to 2030, to $26.99 Mn by 2030.
A pacemaker is a small device designed to treat certain types of arrhythmias. Arrhythmias can cause the heart to beat too fast (tachycardia), too slow (bradycardia), or irregularly. When the heart beats too slowly, it can lead to inadequate oxygen supply to the body and brain, resulting in symptoms like lightheadedness, fatigue, fainting spells, and shortness of breath. Pacemakers work by sending electrical pulses to maintain a normal heart rate and rhythm. Additionally, they can help synchronize the heart chambers, improving the efficiency of blood pumping, which is particularly beneficial for those with heart failure.
Cardiovascular disease (CVD) is the leading cause of death globally. In UAE, CVD accounts for 40% of all deaths. Heart attacks in UAE patients occur 10-15 years earlier than in Western countries, with over 50% of acute coronary syndrome cases involving patients under the age of 55. The market is driven by significant factors like the high prevalence of cardiovascular diseases, advancements in technology, and government support. However, high cost, limited awareness, and risk of complications restrict the growth and potential of the market.
Prominent players in this field include Abbott Laboratories, Biotronik, Boston Scientific, and Medtronic among others.
Market Dynamics
Market Growth Drivers
High Prevalence of CVD: CVD is a leading cause of death in the UAE, responsible for 40% of all fatalities. This high incidence of heart disease drives demand for pacemakers, as they are essential for managing and treating heart conditions effectively.
Advancements in Technology: The growth of the UAE pacemaker market is being propelled by the introduction of advanced technologies, including MRI-compatible and leadless pacemakers. These innovations enhance treatment options and patient outcomes, thereby increasing the demand for pacemakers.
Government Support: Significant investments in healthcare infrastructure and government initiatives aimed at enhancing healthcare services promote the adoption of advanced medical devices, such as pacemakers. This drives market growth in the UAE by improving access to and the quality of cardiac care.
Market Restraints
High Cost: The high cost of pacemaker implantation procedures can restrict access and adoption, particularly among lower-income segments of the population. This financial barrier limits the market growth for cardiac pacemakers in the UAE, as a significant portion of the population may be unable to afford these essential treatments.
Limited Awareness: Limited awareness of pacemaker benefits and apprehensions about surgical procedures can impede adoption among patients and healthcare providers. This acts as a market restraint in the UAE by reducing the acceptance and utilization of pacemakers, thereby hindering market growth.
Risk of Complications: The potential complications linked to pacemaker implantation can affect the decision-making of both patients and physicians, leading to decreased market demand. This serves as a market restraint in the UAE by discouraging some individuals from opting for pacemaker procedures, thus limiting overall market growth.
Regulatory Landscape and Reimbursement Scenario
Cardiac pacemakers are categorized as Class IV medical devices in the UAE, indicating the highest risk level. All Class IV devices, including pacemakers, must obtain full regulatory approval and registration with the UAE Ministry of Health and Prevention (MOHAP) before importation, distribution, or usage. The approval process requires submitting comprehensive technical documentation, clinical evidence, and conformity assessments to prove the device's safety and effectiveness. Public hospitals operated by Abu Dhabi Health Services Company (SEHA) in Abu Dhabi and the Dubai Health Authority (DHA) frequently provide subsidized or discounted pacemaker implantation services for eligible UAE citizens and residents. The UAE government implements initiatives and programs aimed at enhancing affordability and accessibility to pacemaker implantation. These efforts may involve financial assistance, installment payment options, and charitable healthcare options for individuals requiring support. Additionally, many private health insurance plans in the UAE offer partial coverage for pacemaker implantation, with specific coverage terms varying among policies.
Competitive Landscape
Key Players
Here are some of the major key players in the Cardiac Pacemaker Market:
- Abbott Laboratories
- Biotronik
- Boston Scientific
- Medtronic
- Edwards Lifesciences
- Cardinal Health
- Siemens Healthineers
- OSYPKA Medical
- Lepu Medical
- Oscor Inc
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UAE Cardiac Pacemakers Market Segmentation
By Product Type
- Single chamber
- Dual chamber
- Bi ventricular
By Application
- Bradycardia
- Tachycardia
- Other Arrhythmia
By End Users
- Hospitals
- Ambulatory Surgical Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































