UAE Alzheimer’s Therapeutics Market Analysis
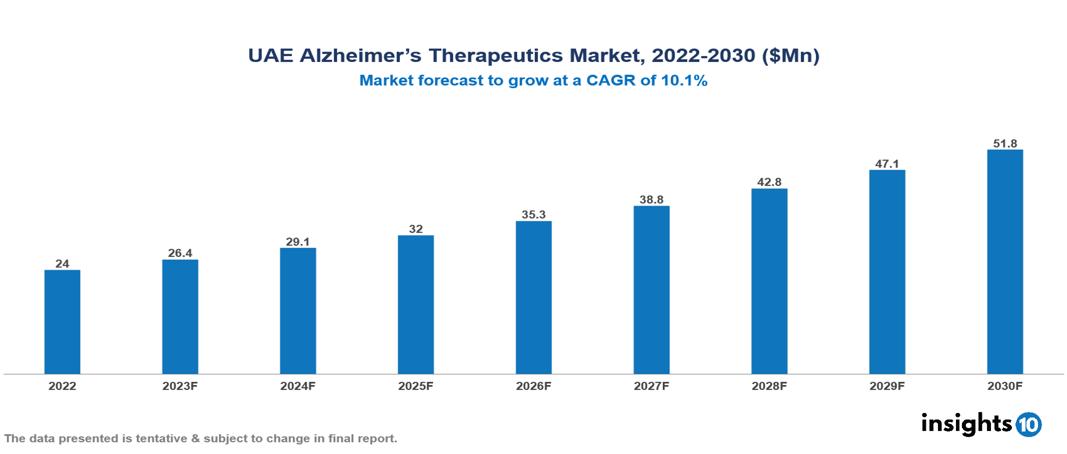
UAE alzheimer’s therapeutics market valued at $24 Mn in 2022, projected to reach $52 Mn by 2030 with a 10.1% CAGR. As the population ages, the prevalence of Alzheimer's disease rises, driving demand for Alzheimer's treatment drugs, one of the major factors driving the market's growth. Leading pharmaceutical companies that are currently working in the market are Pfizer, Eisai, Janssen, Biogen, Roche, Novartis, Lundbeck, Otsuka Pharmaceutical, Teva Pharmaceutical and Sun Pharmaceutical.
Buy Now

UAE Alzheimer’s Therapeutics Market Executive Summary
UAE alzheimer’s therapeutics market valued at $24 Mn in 2022, projected to reach $52 Mn by 2030 with a 10.1% CAGR.
Alzheimer's is a neurological condition that impairs behavior, memory, and mental functions. It usually starts slowly, gets worse with time, and makes it harder for the person to perform daily tasks. Nerve cells in Alzheimer's disease die as a result of abnormal brain alterations such as plaque and tangle buildup. Alzheimer's disease does not currently have a cure. On the other side, some drugs can increase the quality of life and assist manage symptoms. By controlling certain chemicals in the brain, these medications including donepezil, rivastigmine, and memantine alleviate memory and cognitive issues. Furthermore, maintaining a healthy lifestyle, engaging in social and cognitive activities, and fostering a supportive environment are examples of non-pharmacological techniques that may improve overall illness management.
In the United Arab Emirates, the prevalence of dementia in those 65 years of age and older is 4.2%, meaning that 85,000 people were affected by the disease. The aging population will be the cause of the expected surge in cases by 2050, with a stunning 1,795% increase over current levels. Medications like acetylcholinesterase inhibitors such as Aricept (donepezil), Exelon (rivastigmine), and Reminyl (galantamine) as well as the NMDA receptor antagonist Memantine (Namenda) are available for Alzheimer's patients in the United Arab Emirates. The recently licensed medication Aduhelm (aducanumab) is a noteworthy addition to the toolbox because it is particularly made to target amyloid plaques, a hallmark of Alzheimer's disease. Outside of medication-based therapies, non-pharmaceutical methods are also very important. One such method is cognitive stimulation therapy, which involves the brain in tasks that improve thinking, memory, and everyday functioning. Nonpharmacological treatments also include social support, physical activity, and a focus on lifestyle.
The celebration of National Alzheimer's Awareness Month and initiatives like the Alzheimer's Support Group UAE are essential for raising awareness and encouraging early diagnosis. A collaborative environment for knowledge exchange is made possible by the synergy between pharmaceutical corporations, healthcare providers, and patient advocacy groups, which ultimately improves the quality of care.
To further research and development efforts related to Alzheimer's disease, the UAE government continues to collaborate with organizations such as Emirates Health Services and Sheikh Khalifa Medical City.
Market Dynamics
Market Growth Drivers
Entry of New Disease-Modifying Therapies: With the recent approval of Biogen's Aduhelm and Eisai's Leqembi, along with promising new medications like Eli Lilly's Donanemab and Genentech's Gantenerumab, there is hope for slowing the course of the disease and maybe modifying the underlying pathology. These new medicines, in contrast to existing symptomatic therapies, target a more comprehensive unmet requirement. Because of these advancements in treatment, the industry is still expanding. Treatment innovations like this help to sustain the market's continuous growth. Improvements in treatment such as these support the market's ongoing expansion.
Increasing Aging Population: The aging population increases the vulnerability to Alzheimer's disease, increasing the number of people who are at risk. The market for drugs used to treat Alzheimer's disease is gradually expanded by this demographic shift.
Increased Awareness and Early Diagnosis: More people are seeking therapy for Alzheimer's because of increased public knowledge of the illness and advancements in testing technology, which facilitates early diagnosis. The window of opportunity for potential improvement is extended when actions involving prospective disease-modifying medications are started early.
Market Restraints
Lack of Awareness and Stigma: The enduring societal stigma attached to dementia and Alzheimer's disease prevents many people from obtaining a diagnosis and treatment, even despite the increasing number of awareness initiatives. This stigma is a major obstacle that limits the market's ability to reach a broader demographic.
High Drug Costs and Affordability Concerns: For many patients, access to Alzheimer's medications is severely hampered by the treatments' outrageous cost. This problem is especially significant in the UAE since the insurance industry's coverage of these medications is currently developing. Co-payments, even in the case of insurance, can place a significant financial strain on patients and their families.
Limited Efficacy and Uncertain Long-Term Benefits: The effectiveness of current drugs, including Leqembi and Aduhelm, in slowing down cognitive deterioration is questionable, and their long-term sustainability is also questionable. Hesitancy is caused by several factors, including the lack of a clear remedy or solid proof that the disease has improved.
Healthcare Policies and Regulatory Landscape
The Ministry of Health and Prevention (MOHAP) is in charge of regulatory authorities and healthcare policies for medications used to treat Alzheimer's disease in the United Arab Emirates (UAE). The MOHAP is in charge of overseeing and controlling all aspects of healthcare, including the national approval and monitoring of pharmaceuticals. The MOHAP Drug Control Department conducts a comprehensive assessment procedure before approving and registering medications for the treatment of Alzheimer's disease. For regulatory review, pharmaceutical businesses must provide detailed information about the quality, safety, and effectiveness of their products. In the UAE, these medications are sold and supplied after receiving approval.
Competitive Landscape
Key Players
- Pfizer
- Eisai
- Janssen
- Biogen
- Roche
- Novartis
- Lundbeck
- Otsuka Pharmaceutical
- Teva Pharmaceutical
- Sun Pharmaceutical
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UAE Alzheimer’s Therapeutics Market Segmentation
By Type
- Early-Onset Alzheimer's
- Late-Onset Alzheimer's
- Familial Alzheimer's disease
By Drug Name
- Donepezil
- Rivastigmine
- Memantine
- Galantamine
- Manufactured a combination of memantine and donepezil
By Drug Class
- Cholinesterase Inhibitors
- NMDA Receptor Antagonists
- Manufactured Combination
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital pharmacies
- Drug stores
- Retail pharmacies
- Online pharmacies
- Other distribution channel
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































