Turkey ECG Equipments Market Analysis
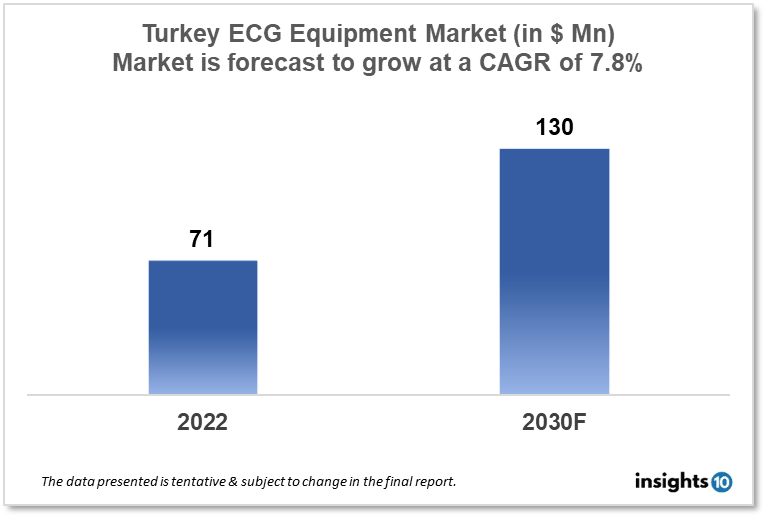
Turkey's ECG Equipment Market is expected to witness growth from $71 Mn in 2022 to $130 Mn in 2030 with a CAGR of 7.80% for the forecasted year 2022-30. The demand for healthcare services and ECG equipment is rising in Turkey as a result of the country's older population. The market is segmented by product type and by end user. Some key players in this market include Ersamed Medikal, Acibadem Labmed, Megasan Medikal, Johnson & Johnson, Philipps Healthcare, Medtronic, GE Healthcare, and Nihon Kohden.
Buy Now

Turkey ECG Equipment Healthcare Market Executive Analysis
Turkey's ECG Equipment Market is expected to witness growth from $71 Mn in 2022 to $130 Mn in 2030 with a CAGR of 7.80% for the forecasted year 2022-30. Turkish healthcare spending totaled $14.38 billion in 2022, a 24.3% increase over 2019. The total amount spent on health increased by 26.3% to $10.48 billion. Spending on healthcare in the private sector was anticipated to reach $2.70 billion, a rise of 17.3%. In contrast to the private sector, which accounted for 20.8% of health expenses in 2020, the federal government accounted for 79.2% of them.
In Turkey, there were over 100,000 occurrences of acute myocardial infarction, also known as heart attacks, annually in 2021. Turkey has a 20% prevalence of cardiovascular disease. Electrocardiography (ECG) equipment, often known as ECG equipment, is frequently used in Turkey to diagnose and track a variety of heart disorders. To diagnose heart diseases such as arrhythmias, myocardial infarction, and heart failure, ECG equipment is frequently employed. The apparatus can identify damaged heart muscle, pick up on aberrant cardiac rhythms, and aid in the diagnosis of congenital heart abnormalities. Rapid readings from ECG devices are helpful in emergencies. As a result of the equipment's speedy diagnosis of diseases like heart attacks, medical personnel can start treating patients right away. ECG equipment comes in a variety of shapes and sizes, including portable and handheld ones. This makes it simple to utilize in a variety of situations, such as clinics, hospitals, and ambulances.
Market Dynamics
Market Growth Drivers
The demand for healthcare services and ECG equipment is rising in Turkey as a result of the country's older population. Heart diseases are more common in the elderly, which raises the need for ECG equipment. The Turkish government has put in place a number of initiatives to enhance the healthcare system in the nation. These programmes include promoting preventative healthcare, building more hospitals and clinics, and investing in the infrastructure of the healthcare industry. As a result, there will probably be a rise in the demand for ECG equipment. The Turkish government has been spending more money on healthcare recently, which is probably going to raise demand for ECG equipment. Hospitals and clinics are likely to invest in new medical gadgets, such as ECG equipment, as healthcare spending rises.
Market Restraints
In Turkey, several well-known brands already control a large portion of the ECG equipment industry. Gaining momentum and increasing their market share may be difficult for new competitors. The use of ECG equipment in Turkey may be constrained by the fact that some medical practitioners there may not be aware of its advantages. To promote knowledge and use of ECG equipment, manufacturers may need to make investments in educational and training initiatives.
Competitive Landscape
Key Players
- Ersamed Medikal (TR)
- Acibadem Labmed (TR)
- Megasan Medikal (TR)
- Johnson & Johnson
- Medtronic
- Philipps Healthcare
- GE Healthcare
- Nihon Kohden
Healthcare Policies and Regulatory Landscape
The Turkish Ministry of Health (MoH) and the Turkish Medicines and Medical Devices Agency (TTCK) are in charge of regulating the ECG equipment healthcare system in Turkey. The main law that controls the approval, registration, and distribution of medical devices, including ECG equipment, in Turkey, is the Medical Device Regulation (MDR). The MDR, which is based on the Medical Device Regulation (EU MDR) of the European Union, is implemented by the TTCK. Before they may be sold in Turkey, all medical products, including ECG equipment, must pass a conformity evaluation. The evaluation is done by a Notified Body that has been granted permission by the TTCK to carry out the evaluation and grant the CE mark, which denotes compliance with the MDR.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
ECG Equipment Market Segmentation
By Product Type (Revenue, USD Billion):
- Holter Monitors
- Resting ECG Machines
- Stress ECG Machines
- Event Monitoring Systems
- ECG Management Systems
- Cardiopulmonary Stress Testing Systems
By End User (Revenue, USD Billion):
- Hospitals and Clinics
- Diagnostic Centres
- Ambulatory Services
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































