Turkey Diabetes Therapeutics Market Analysis
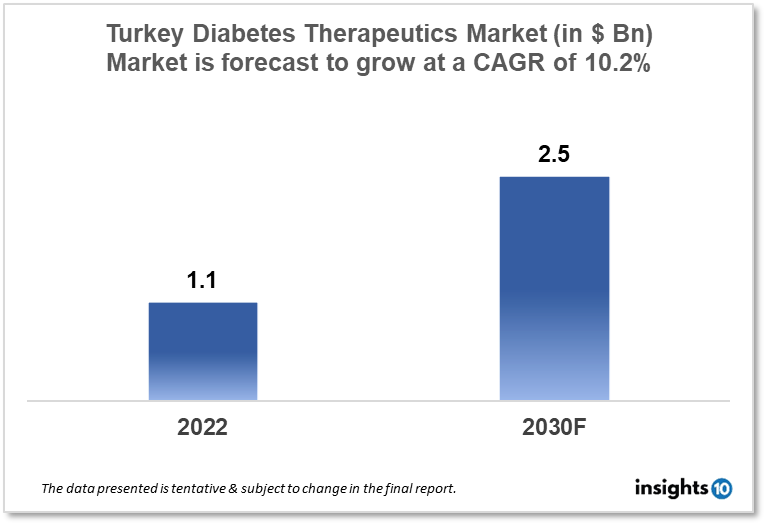
Turkey's diabetes therapeutics market is expected to witness growth from $1.1 Bn in 2022 to $2.5 Bn in 2030 with a CAGR of 10.2% for the forecasted year 2022-30. The rising awareness about diabetes and the supportive government policies to promote diabetes in Turkey are the major market drivers. The Turkey diabetes therapeutics market is segmented by type, application, drug, route of administration, and distribution channel. Nobel İlaç, Koçak Farma, and GlaxoSmithKline are the major players in the Turkey diabetes therapeutics market.
Buy Now

Turkey Diabetes Therapeutics Market Executive Analysis
Turkey's diabetes therapeutics market is at around $1.1 Bn in 2022 and is projected to reach $2.5 Bn in 2030, exhibiting a CAGR of 10.2% during the forecast period. In the 2023 budget, the Turkish Ministry of Health increased the funds allotted for preventative health care by eight times, totaling $1.78 Bn. Additionally, it was mentioned that hospitals outfitted with the most cutting-edge equipment are being placed into operation one at a time, and the Ministry offers services through more than 14,000 healthcare facilities, including 953 hospitals and 8,000 family health centers.
With more than six Mn individuals, Turkey has the third-largest diabetes population in the world and the largest in Europe. The International Diabetes Federation (IDF) reports that the prevalence rate varies between 11 and 14 % of the population, but that the proportion of people without a diabetes diagnosis is 38%. A hormone called insulin aids in controlling the body's blood sugar levels. People with type 1 and type 2 diabetes who need to take insulin to control their blood sugar levels frequently utilize this medication. Biguanides, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists are a few of the kinds of oral drugs used to treat type 2 diabetes. GLP-1 receptor agonists and amylin mimetics are two injectable drugs that help persons with type 2 diabetes control their blood sugar levels.
Market Dynamics
Market Growth Drivers
Turkey is experiencing a rise in diabetes awareness, which is projected to increase the demand for diabetic medicines. Government agencies and healthcare professionals are attempting to inform the public about the value of diabetes control and early identification. With a focus on enhancing access to healthcare services and managing chronic diseases like diabetes, the Turkish government has been expanding its healthcare spending in recent years. The Turkey diabetes therapeutics market is anticipated to grow as a result of this rise in healthcare spending.
Market Restraints
Although the Turkish government has been spending more money on healthcare, access to services is still restricted in some places, especially in rural areas. By reducing the number of people who have access to diabetic medicines, this limited healthcare access may impede market expansion. The high prevalence of diabetes in Turkey may be influenced by cultural and lifestyle elements like the country's high obesity rate and unhealthful diet habits. These elements may also reduce the efficacy of diabetes treatments and restrain market expansion. The approval and adoption of new diabetes medicines could be slowed or delayed by the complexity of the Turkish pharmaceutical regulatory environment. As a result, there may be fewer newer therapies available, which could slow Turkey's diabetes therapeutics market expansion.
Competitive Landscape
Key Players
- Abdi İbrahim (TUR)
- Deva Holding (TUR)
- İlko İlaç (TUR)
- Nobel İlaç (TUR)
- Koçak Farma (TUR)
- Glaxosmithkline
- Johnson Johnson
- Merck
- Novartis
- Novo Nordisk
- Sanofi
- Takeda Pharmaceutical
Healthcare Policies and Regulatory Landscape
The primary regulating and accountable body for medications, biologicals, and medical devices in Turkey is the Ministry of Health (the "Ministry"). Pharmaceutical items cannot be introduced to the market without first receiving a license from the Ministry. A request for a license must be made to the Ministry along with the supporting documentation outlined in the Licensing Regulation on Medicinal Products. The applicant must carry out clinical trials, toxicological and pharmacological investigations, and more before filing a licensing application. The process for carrying out clinical trials is described in the Regulation on Clinical Research. A clinical trial dossier must be submitted concurrently to the Turkish Medicines and Medical Devices Agency (TTCK) and the Ethics Committee (designated by the Ministry) as the first step in the application process.
To ensure that medical items are made available to customers on fair terms and to promote a more effective and long-lasting healthcare system, the Ministry of Health establishes the maximum prices and applies pricing measures. Turkish pharmaceutical costs are based on reference prices from five reference countries, all of which are members of the European Union, as defined by the Ministry of Health. The lowest warehouse sales price for authentic and authorized goods (excluding discounts) in the reference nations is referred to as the reference price. The lower price shall be taken into account as the reference price if, however, the country where the product is produced or imported is not one of the reference countries but the warehouse sales price of the pertinent product is less than the reference prices in the reference countries.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Diabetes Therapeutics Segmentation
By Type (Revenue, USD Billion):
- Diabetes 1
- Diabetes 2
By Application (Revenue, USD Billion):
- Preventive
- Prediabetes
- Nutrition
- Obesity
- Lifestyle Management
- Treatment/Care
- Diabetes
- Smoking Cessation
- Musculoskeletal Disorders
- Central Nervous System Disorders
- Cardiovascular Disease
- Medication Adherence
- Chronic Respiratory Disorders
- Gastrointestinal Disorders
- Rehabilitation
- Substance Use Disorders & Addiction Management
By Drug (Revenue, USD Billion):
- Oral Anti-diabetic Drugs
- Insulin
- Non-insulin Injectable Drug
- Combination Drug
By Route of Administration (Revenue, USD Billion):
- Oral
- Subcutaneous
- Intravenous
By Distribution Channel (Revenue, USD Billion):
- Online Pharmacies
- Hospital Pharmacies
- Retail Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
Nobel İlaç, Koçak Farma, and GlaxoSmithKline are the major players in the Turkey diabetes therapeutics market.
The Turkey diabetes therapeutics market is expected to grow from $1.1 Bn in 2022 to $2.5 Bn in 2030 with a CAGR of 10.2% for the forecasted year 2022-2030.
The Turkey diabetes therapeutics market is segmented by type, application, drug, route of administration, and distribution channel.








































