Turkey Diabetes Devices Market Analysis
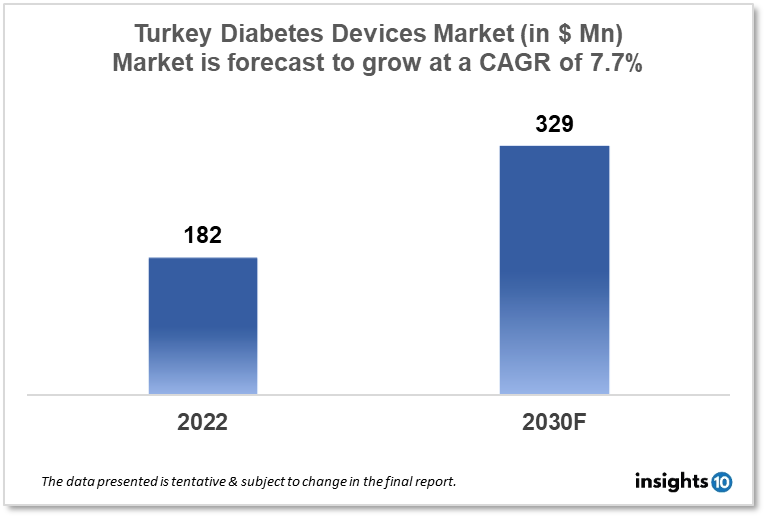
The Turkey Diabetes Devices Market is expected to witness growth from $182 Mn in 2022 to $329 Mn in 2030 with a CAGR of 7.70% for the forecasted year 2022-2030. Affordable healthcare options, reasonably lax regulatory requirements, and cost-effective labour are a few of the factors driving diabetic device manufacturers to grow their businesses in Turkey. The market is segmented by type and by the end user. Some key players in this market include Megasan Medikal, Kare Medical, Johnson & Johnson, Philipps Healthcare, Medtronic, Roche and Dexcom.
Buy Now

Turkey Diabetes Devices Healthcare Market Executive Analysis
The Turkey Diabetes Devices Market is at around $182 Mn in 2022 and is projected to reach $329 Mn in 2030, exhibiting a CAGR of 7.70% during the forecast period. 2020 observed a 24.3% increase in Turkey's overall healthcare spending to $13.7 billion. Overall health expenditure increased by 26.3%, or $10.48 billion. The $2.70 billion in anticipated private healthcare spending indicates an increase of 17.3%. In 2020, the federal government paid 79.2% of all health-related expenses compared to the private sector's 20.8% portion.
In Turkey, approximately 5.5 million individuals between the ages of 20 and 79 had diabetes in 2021, which means a prevalence rate of 6.9%. Turkish individuals aged 35 to 74, with type 2 diabetes had an incidence rate of 7.5 per 1,000 person-years. For kids and adolescents aged 0 to 19 years, the incidence rate of type 1 diabetes is 12.8 per 1,000 person-years. In Turkey, a variety of diabetes devices is available to help people with the disease successfully control their condition. Blood glucose levels must be measured using blood glucose metres in order to effectively control diabetes. Because blood glucose metres are portable, simple to use, and yield findings quickly, people can modify their diet, exercise routines, and medication as necessary. Insulin is constantly delivered by small devices called insulin pumps through a catheter inserted under the skin. They enable more accurate insulin administration and can be programmed to administer various dosages of insulin throughout the day depending on user requirements. This can help people with diabetes better manage their blood sugar levels, lower their risk of complications, and enhance their quality of life.
Market Dynamics
Market Growth Drivers
Turkey is experiencing an increase in the number of diabetics, which is driving up demand for diabetes devices. Affordable healthcare options, reasonably lax regulatory requirements, and cost-effective labour are a few of the factors driving diabetic device makers to grow their businesses in Turkey. The introduction of high-end insulin pumps and pens, as well as other technological developments in diabetes devices, are driving up demand for these products in the Turkish healthcare industry. Leading manufacturers are concentrating on technological advancements and the creation of cutting-edge goods in order to capture a sizeable portion of the Turkish market. There is an increasing need for diabetes devices in Turkey as people become more aware of the importance of controlling diabetes and the benefits of using diabetes devices. As more people look for these devices to enhance their health outcomes, this is anticipated to propel development in the market for diabetes devices.
Market Restraints
In Turkey, the restricted reimbursement for diabetes devices may prevent some people from using them. This may reduce consumer desire for devices and harm manufacturers' prospects for expansion. Some people in Turkey may favour traditional techniques over contemporary diabetes devices due to cultural differences in attitudes towards diabetes management. This may reduce consumer desire for devices and harm manufacturers' prospects for expansion.
Competitive Landscape
Key Players
- Megasan Medikal (TR)
- Kare Medical (TR)
- Johnson & Johnson
- DarioHealth
- Medtronic
- Philipps Healthcare
- Dexcom
Recent Notable Deals
October 2022: To distribute its diabetes care devices in Turkey, medical device manufacturer Medtronic announced in October 2022 a partnership with Birgi Mefar Group, a Turkish healthcare technology firm.
2021: A licencing agreement was signed by the Turkish pharmaceutical firm Sanovel and the US-based medical technology company Senseonics to distribute the Eversense continuous glucose monitoring system in Turkey.
Healthcare Policies and Regulatory Landscape
Governmental organisations such as the Ministry of Health (MoH) and the Turkish Medicines and Medical Devices Agency (TTCK) are in charge of regulating and enforcing healthcare policies in Turkey's market for diabetes devices. Before they can be sold in Turkey, diabetes devices must obtain TTCK marketing authorization. The device's safety and effectiveness are ensured through a rigorous process of clinical trials and technological evaluations. The Turkish Social Security Institution (SGK) is in charge of choosing the amounts that should be reimbursed for diabetes equipment. Manufacturers must file for reimbursement before their devices can be covered by the SGK, and the rates of reimbursement are reviewed on a regular basis. The norms and laws of Turkey must be followed for diabetes device imports. The TTCK and other pertinent authorities must also grant the required licences and approvals to manufacturers.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Diabetes Devices Market Segmentation
By Type (Revenue, USD Billion):
The market is divided into blood glucose monitoring systems, insulin delivery systems, and mobile applications for managing diabetes within the type segment. Due to its convenience, ease of use, and usefulness in providing patients and healthcare professionals with real-time insights regarding diabetic conditions for integrated diabetes management, the segment for diabetes management mobile applications is anticipated to grow at the highest rate during the forecast period. Bare-metal Stents
- Blood glucose monitoring systems
- Self-monitoring blood glucose monitoring systems
- Continuous glucose monitoring systems
- Test strips/Test papers
- Lancets/Lancing Devices
- Insulin delivery Devices
- Insulin pumps
- Insulin pens
- Insulin syringes and needles
- Diabetes management mobile applications
By End User (Revenue, USD Billion):
The diabetes market is divided into hospitals & specialty clinics and self & home care, based on the end user.
- Hospitals & Specialty Clinics
- Self & Home Care
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































