Sweden Bioinformatics Market Analysis
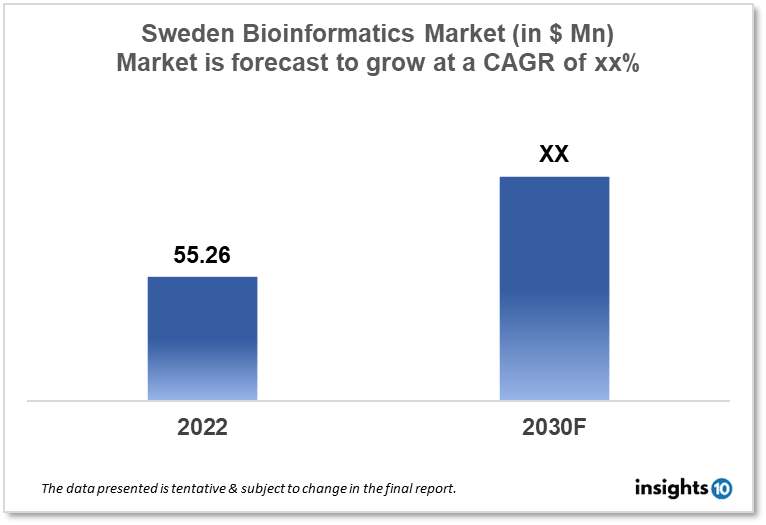
Sweden's Bioinformatics market is projected to grow from $55.26 Mn in 2022 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2022-30. The main factors driving the growth would be a high level of R&D investments, a strong healthcare system, and increased digitalization. The market is segmented by technology and by application. Some of the major players include Agilent Technologies, IBM Life Sciences, Qiagen, Genomic Labs, and Lab Leader AB.
Buy Now

Sweden Bioinformatics Market Executive Summary
Sweden's Bioinformatics market is projected to grow from $55.26 Mn in 2022 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2022-30. In Sweden, local taxes, direct transfers from the national government, subsidies to the regions for outpatient medications, and particular national programs make up the majority of the funding for health spending. Sweden spent 10.9% of its GDP on health in 2019, which was higher than the EU average of 9.9% and ranked third among EU nations.
Bioinformatics is the study of biology with information technology. In bioinformatics, various approaches are used, such as data management, data mining, data warehousing, and data generation. Statistical techniques and data processing algorithms are provided by bioinformatics software and tools as integrated solutions for applications including next-generation sequencing, modeling of genomic and proteomic structure, and three-dimensional drug discovery. The Bioinformatics market in Europe holds the second-largest share of the global market.
Market Dynamics
Market Growth Drivers
The Sweden bioinformatics market is expected to be driven by factors such as a high level of R&D investments, a strong healthcare system, and increased digitalization. Sweden is known for its high level of healthcare digitalization, which lays a solid platform for the expansion of the bioinformatics sector. This comprises an effective healthcare system, a system for keeping track of electronic health records that is well-developed, and a high penetration of digital devices and the internet.
Market Restraints
The high cost of developing and implementing bioinformatics solutions and the lack of standardization in the field and high cost of developing and implementing bioinformatics solutions can limit the growth of the industry. Also, Sweden's bioinformatics market is modest compared to other European and North American nations. As market size can influence the amount of investment and the number of businesses in a certain industry, this may limit the market's potential for expansion.
Competitive Landscape
Key Players
- Agilent Technologies
- IBM Life Sciences
- Qiagen
- Genomic Labs
- Lab Leader AB
Healthcare Policies and Regulatory Landscape
Sweden has a strong healthcare system and places considerable importance on bioinformatics innovation. Government initiatives to encourage business and academic cooperation as well as financing for research and development have all been put in place to help the development of the bioinformatics sector. The aim of the Swedish government is for the nation to rank among the top digital nations in the world. One industry where the government has made significant digitization investments in healthcare, is in an effort to raise the standard and accessibility of care.
In Sweden, medical products are governed by the Swedish Medical Products Agency (MPA). Prior to being commercialized in Sweden, medical items must pass the MPA's quality, safety, and efficacy tests. Further, Sweden develops medicinal products in accordance with standards established by the European Union (EU) and the European Medicines Agency (EMA). This covers regulations for data security and privacy as well as recommendations for applying artificial intelligence to healthcare.
Reimbursement Scenario
The Swedish National Board of Health and Welfare, in conjunction with the Swedish Association of Local Authorities and Regions (SALAR) and the Swedish Association of Health Professionals, establishes a reimbursement policy for healthcare products and services in Sweden. The reimbursement process normally entails a number of processes, such as a clinical evaluation of the good or service, a cost-effectiveness analysis, and a decision on whether to reimburse the good or service by the National Board of Health and Welfare. The availability of alternative therapies, the cost-effectiveness of the product or service, and its clinical efficacy are all factors taken into account when determining whether to pay a provider. However, reimbursement procedures for bioinformatics-related goods and services are not well-established since the subject is still relatively young.
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Bioinformatics Market Segmentation
By Technology (Revenue, USD Billion):
- Knowledge Management Tools
- Bioinformatics Platforms
- Bioinformatics Services
By Application (Revenue, USD Billion):
- Metabolomics
- Molecular phylogenetics
- Transcriptomics
- Chemoinformatic & drug design
- Genomics
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































