Spain Prurigo Nodularis Therapeutics Market Analysis
Spain Prurigo Nodularis Therapeutics Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. Growing research and development activities by key companies and rising government funding in developed areas would be the main drivers of the market demand for prurigo nodularis treatments. Major global players in Prurigo Nodularis Therapeutics Market are Novartis AG (Switzerland), AstraZeneca (UK), Merck & Co., Inc. (US), Sumitomo Corporation (Japan), Allergan (Ireland), LEO Pharma A/S (Denmark), Cipla Inc. (US), Eli Lilly and Company (US), Bayer AG (Germany), Abbott (US), Pfizer Inc. (US), GlaxoSmithKline plc (UK), Johnson & Johnson Private Limited (US), Teva Pharmaceutical Industries Ltd.(Jerusalem), Sun Pharmaceutical Industries Ltd. (Mumbai), Aurobindo Pharma (Hyderabad), Lupin (Mumbai), Bausch Health Companies Inc. (Canada), F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (US)
Buy Now

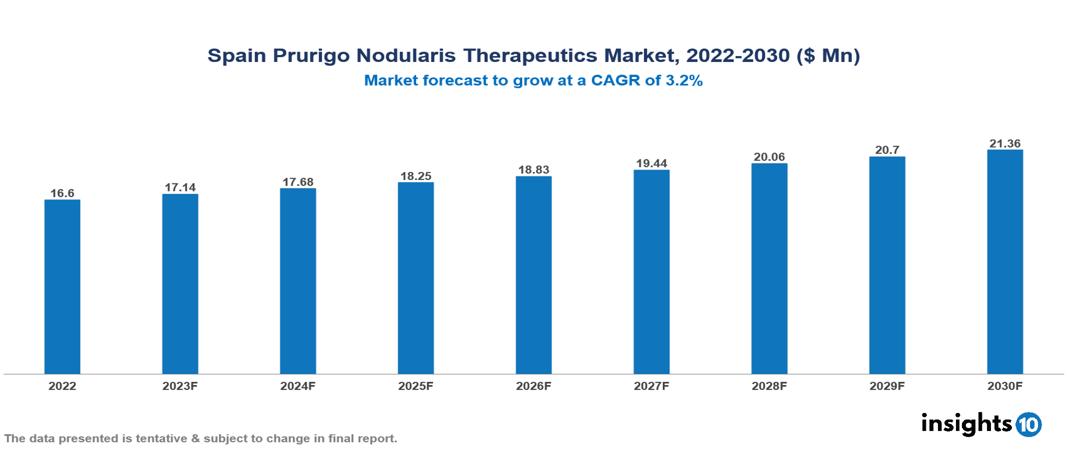
Spain Prurigo Nodularis Therapeutics Market Analysis Summary
Spain Prurigo Nodularis Therapeutics Market is valued at around $16.6 Mn in 2022 and is projected to reach $21.36 Mn by 2030, exhibiting a CAGR of 3.2% during the forecast period 2023-2030.
Red, itchy, and occasionally painful pimples appear on the body as a result of a skin ailment known as prurigo nodularis. Numerous studies have revealed that nickel, which is contained in coins, jewellery, and clothing, as well as laundry detergent, dogs, and high pollen concentrations, may all be the causes even if the exact cause of this ailment is unknown. Steroids are routinely administered intravenously or topically as therapies. Phototherapy or systemic immunosuppressive medications are frequently used in more severe cases or situations that are treatment-resistant. Even though both thalidomide and lenalidomide can be used in severe situations; their toxicity makes them less desirable. Neurokinin-1 receptor antagonists and opioid receptor antagonists are two effective treatments for prurigo nodularis in contrast to thalidomide or lenalidomide. Research advancements related to the treatment of prurigo nodularis will create new prospects for the market's growth rate. Pharmaceutical companies are constantly concentrating on the development of new drugs and therapies in order to deliver the best therapeutic outcomes.
Growing research and development activities by key companies and rising government funding in developed areas would be the main drivers of the market demand for prurigo nodularis treatments. Major global players in Prurigo Nodularis Therapeutics Market are Novartis AG (Switzerland), AstraZeneca (UK), Merck & Co., Inc. (US), Sumitomo Corporation (Japan), Allergan (Ireland), LEO Pharma A/S (Denmark), Cipla Inc. (US), Eli Lilly and Company (US), Bayer AG (Germany), Abbott (US), Pfizer Inc. (US), GlaxoSmithKline plc (UK), Johnson & Johnson Private Limited (US), Teva Pharmaceutical Industries Ltd.(Jerusalem), Sun Pharmaceutical Industries Ltd. (Mumbai), Aurobindo Pharma (Hyderabad), Lupin (Mumbai), Bausch Health Companies Inc. (Canada), F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (US).
Market Dynamics
Market Drivers
- high incidence of chronic illnesses
The rise in the prevalence of chronic diseases such as diabetes, chronic renal failure, lymphoma, lichen planus, HIV, neurological disorders, and others would be a major factor in the market's expansion of growth rate.
- Increasing infrastructure spending for healthcare
- The market for prurigo nodularis treatments will grow as a result of greater awareness-raising efforts by governmental and non-governmental organisations, as well as a growth in the elderly population. Due to additional variables including a high disposable income and a constantly changing way of life, the market for prurigo nodularis therapies will expand more quickly. The incidence of prurigo nodularis treatment in developing countries and increased focus on orphan medication research will both enhance market growth.
Market Restraints
Due to high treatment costs and a lack of infrastructure in low-income countries, the prurigo nodularis treatment market will expand slowly. Additionally, the market growth for prurigo nodularis treatments may be constrained by a lack of understanding of the treatment procedure. Additional commercial difficulties will result from patients' lower levels of awareness and the lack of approved targeted medicines or diagnoses.
Key players
Galderma Almirall Novartis Pfizer Johnson & Johnson UCB Pharma Sanofi Astellas Pharma Meda Pharma Almirall1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Spain Prurigo Nodularis Therapeutics Market
By Treatment Type
- Medication
- Cryotherapy
- Phototherapy
- Pulsed Dye Laser
- Others
By Diagnosis
- Skin Biopsy
- Blood Tests
- Others
By Route of Administration
- Oral
- Parenteral
- Topical
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































