Spain Nanomedicine Market Analysis
Spain nanomedicine market is projected to grow from $xx Mn in 2022 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2022 - 2030. Shortly, significant advancements and the recent commercialization of goods like mRNA- and liposome-based vaccines are anticipated to fuel the rise of nanomedicines. The major key players in this market are Abbott Laboratories, CombiMatrix Corporation, Celgene Corporation, etc.
Buy Now

Spain Nanomedicine Market Analysis Summary
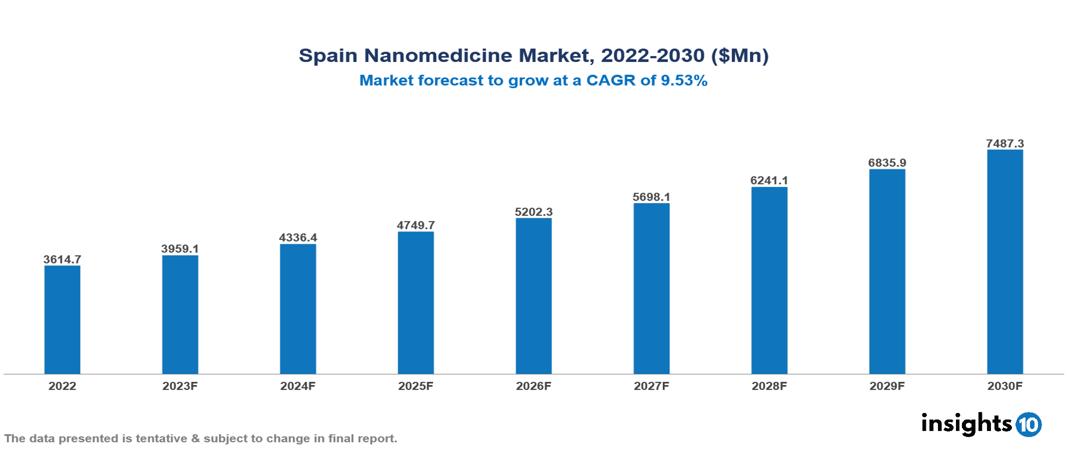
Spain Nanomedicine Market is valued at around $3614.7 Mn in 2022 and is projected to reach $7487.3 Mn by 2030, exhibiting a CAGR of 9.53% during the forecast period 2023-2030.
Due to their small size, nanomaterials have unique physicochemical features that set them apart from their bulk chemical equivalents. These characteristics significantly expand the number of potentials for medication development. The physicochemical characteristics of the nanoformulation have the potential to cross biological barriers, have toxic properties, and have persistence in the environment and the human body. These characteristics can change pharmacokinetics, such as absorption, distribution, elimination, and metabolism. Nanomaterials are manufactured in the pharmaceutical industry using both top-down and bottom-up strategies. By using mechanical or chemical energy, a bulk material is broken down into one or more smaller bits as part of the top-down process. The bottom-up method, on the other hand, begins with atomic or molecular species that allow precursor particles to grow in size through chemical reactions. Shortly, significant advancements and the recent commercialization of goods like mRNA- and liposome-based vaccines are anticipated to fuel the rise of nanomedicines. The widespread ineffectiveness of conventional therapies and the expanding use of drug delivery techniques based on nanotechnology offer additional potential growth prospects for the nanomedicine sector. The major key players in this market are Abbott Laboratories, CombiMatrix Corporation, Celgene Corporation, Nanospectra Biosciences, Inc., GE Healthcare, Johnson & Johnson Services, Inc., Mallinckrodt Pharmaceuticals, Merck & Co., Inc., Pfizer, Inc., Teva Pharmaceutical Industries Ltd., Arrowhead Pharmaceuticals, Inc.
Market Dynamics
Market Growth Drivers
The advantages of nanomedicine in numerous healthcare applications, the emergence of novel drug delivery technologies, and the rise in demand for safe and affordable treatments all contribute to the market's expansion. Additionally, a rise in research and development (R&D) projects on nanorobots in this market sector and sizeable public and private sector investments in clinical trials also contribute to the market's expansion. However, it is anticipated that the lengthy licensing process and hazards connected with nanomedicines (environmental effect) will restrain market expansion. In contrast, the rising out-licensing of nanodrugs and the expansion of healthcare facilities in developing nations are anticipated to present profitable potential for market expansion.
Market Restraints
Nanomedicine is created and produced using expensive, innovative technology and intricate manufacturing procedures. A larger patient population might not have access to nanomedicine because of the high cost of research, development, and manufacturing, especially in poor nations with scarce healthcare resources thus high cost of treatment will hamper the growth of the nanomedicine market.
Competitive Landscape
Key Players
- Abbott Laboratories
- CombiMatrix Corporation
- Celgene Corporation
- Nanospectra Biosciences, Inc.
- GE Healthcare
- Johnson & Johnson Services, Inc.
- Mallinckrodt Pharmaceuticals
- Merck & Co., Inc.
- Pfizer, Inc.
- Teva Pharmaceutical Industries Ltd.
- Arrowhead Pharmaceuticals, Inc.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Spain Nanomedicine Market Segmentation
By Modality
- Diagnostics
- Treatment
By Application
- Drug Delivery
- Diagnostic Imaging
- Vaccines
- Regenerative Medicine
- Implants
- Others
By Indications
- Clinical Oncology
- Infectious Diseases
- Clinical Cardiology
- Orthopedics
- Neurology
- Urology
- Ophthalmology
- Immunology
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































