Spain Digital Biomarkers Market Analysis
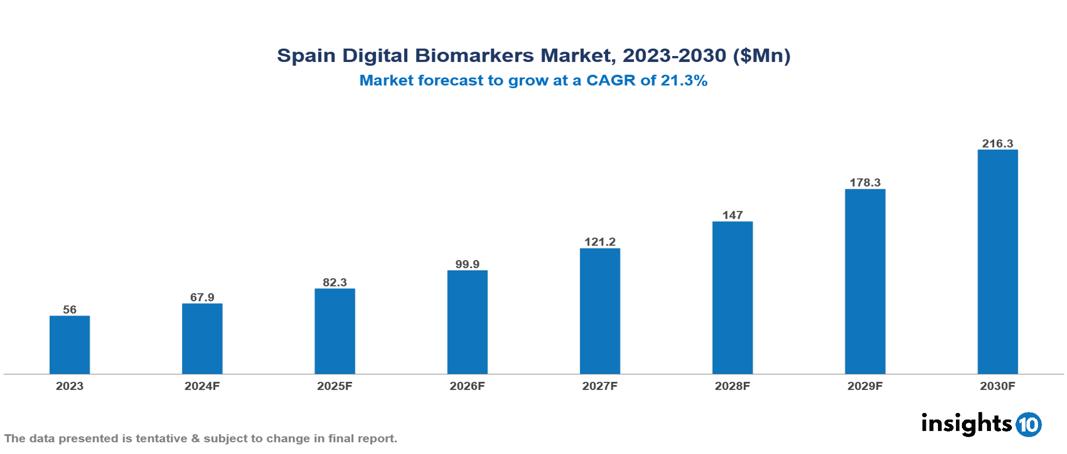
Spain Digital Biomarkers Market was valued at $55.97 Mn in 2023 and is predicted to grow at a CAGR of 21.3% from 2023 to 2030, to $216.26 Mn by 2030. The key drivers of this industry include the high burden of chronic diseases, the advanced healthcare system, and the growing interest in personalized medicine. The industry is primarily dominated by ActiGraph LLC, AliveCor Inc., Koneksa, and Altoida Inc. among others.
Buy Now

Spain Digital Biomarkers Market Executive Summary
Spain Digital Biomarkers Market was valued at $55.97 Mn in 2023 and is predicted to grow at a CAGR of 21.3% from 2023 to 2030, to $216.26 Mn by 2030.
Digital biomarkers are quantifiable physiological and behavioral data collected by digital devices like wearables and smartphones. They provide insights into health, including disease progression and treatment response, enabling continuous monitoring and real-time data collection. These biomarkers are used for various health conditions, such as diabetes, cardiovascular disorders, mental health issues, and neurodegenerative diseases. By tracking metrics like physical activity, heart rate, and sleep patterns, digital biomarkers help clinicians make informed decisions and support research with real-world evidence. As technology advances, their potential to transform healthcare and improve patient outcomes grows.
In Spain, chronic diseases dominate mortality, comprising nearly 90% of all deaths. Hypertension affects about 40% of adults, diabetes impacts approximately 14%, and musculoskeletal disorders, including chronic pain in over 60% of older adults, are prevalent. The WHO noted over 59,000 active clinical trials globally in 2020, underscoring the rising demand for digital biomarkers.
The market therefore is driven by significant factors like the high burden of chronic diseases, advanced healthcare system, and growing interest in personalized medicine. However, cybersecurity threats, the need for standardization, and limited reimbursement restrict the growth and potential of the market.
Prominent players in this field are ActiGraph LLC which specializes in wearable devices and data analytics for physical activity and sleep monitoring, contributing to clinical research through reliable digital biomarkers. AliveCor Inc. excels in portable ECG monitoring and AI-driven cardiac analysis, enhancing early diagnosis and intervention with FDA-cleared devices like KardiaMobile. Other contributors include Koneksa, and Altoida Inc. among others.
Market Dynamics
Market Growth Drivers
High Burden of Chronic Diseases: Spain faces a significant burden from chronic diseases such as diabetes, cardiovascular diseases, and cancer. Digital biomarkers offer critical tools for early detection, continuous monitoring, and effective management of these conditions.
Advanced Healthcare System: Spain's healthcare system emphasizes preventative care and population health management. This proactive approach creates a conducive environment for integrating digital biomarkers into routine healthcare practices. By leveraging digital biomarkers, healthcare professionals can implement personalized health strategies, monitor at-risk populations more effectively, and optimize resource allocation for chronic disease management.
Growing Interest in Personalized Medicine: There is a notable shift towards personalized medicine in Spain, driven by advancements in genomic research and precision healthcare. Digital biomarkers play a crucial role by providing tailored health insights based on individual patient data. These biomarkers enable healthcare professionals to personalize treatment plans, predict disease risks more accurately, and optimize therapeutic outcomes, thereby driving market expansion.
Market Restraints
Cybersecurity Threats: Digital biomarkers, often integrated with mobile apps and cloud-based platforms, are vulnerable to cyber threats. Ensuring robust cybersecurity measures is imperative to safeguard sensitive health information, maintain data integrity, and mitigate risks associated with unauthorized access or data breaches.
Need for Standardization: Standardizing data collection, analysis, and interpretation of digital biomarker data is critical for ensuring consistency and reliability in clinical decision-making. Harmonizing methodologies and establishing clear guidelines for data validation and interpretation can enhance the usability and comparability of digital biomarker insights across healthcare providers and research institutions.
Limited Reimbursement: Despite improvements, reimbursement for digital biomarkers in Spain remains inconsistent. Variations in coverage policies across regions and healthcare settings can hinder widespread adoption. Uncertain reimbursement models pose challenges for healthcare providers and technology developers seeking to invest in and scale digital biomarker solutions, particularly in a cost-conscious healthcare environment.
Regulatory Landscape and Reimbursement Scenario
Spain's regulatory framework for digital biomarkers, overseen by the Spanish Agency for Medicines and Medical Devices (AEMPS), is well-established yet dynamic. AEMPS regulates medical devices, including app-based digital biomarkers categorized under "productos sanitarios basados en aplicaciones." Stakeholders navigating this landscape should consult AEMPS guidelines and the Spanish Data Protection Agency (AEPD) regulations, ensuring compliance with stringent data privacy standards. Moreover, adherence to EU regulations like the Medical Devices Regulation (MDR) and In-Vitro Diagnostics Regulation (IVDR) is essential, reflecting Spain's integration into broader European standards for medical technologies.
In terms of reimbursement, Spain's National Health System (Sistema Nacional de Salud - SNS) offers expanding coverage for digital health solutions incorporating digital biomarkers. Reimbursement eligibility within the SNS depends on demonstrated clinical benefits and alignment with updates to the Public Health Benefits Catalogue (Catálogo Público Prestacional). Additionally, private health insurers in Spain provide varying levels of coverage for digital health innovations, including digital biomarkers, contingent upon individual policies and technological assessments. Monitoring developments across both public and private sectors is crucial for stakeholders aiming to leverage opportunities in Spain's evolving digital biomarkers market.
Competitive Landscape
Key Players
Here are some of the major key players in the Spain Digital Biomarkers Market
- ActiGraph LLC
- AliveCor Inc.
- Koneksa
- Altoida Inc.
- Amgen Inc.
- Biogen Inc.
- Empatica Inc.
- Vivo Sense
- IXICO plc
- Adherium Limited
1. Executive Summary
1.1 Digital Health Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Digital Health Policy in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Spain Digital Biomarkers Market Segmentation
By Type
- Wearable
- Mobile-based Applications
- Sensors
- Others
By Clinical Practice
- Diagnostic digital biomarkers
- Monitoring digital biomarkers
- Predictive and Prognostic digital biomarkers
- Other's (Safety, Pharmacodynamics/ Response, Susceptibility)
By Therapeutic Area
- Cardiovascular and metabolic disorders (CVMD)
- Respiratory disorders
- Psychiatric disorders
- Sleep & Movement Disease
- Neurological disorders
- Musculoskeletal disorders
- Others (Diabetes, Pain Management)
By End-Use
- Healthcare companies
- Healthcare Providers
- Payers
- Others (Patients, caregivers)
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.










































