Spain Cardiac Resynchronization Therapy Market
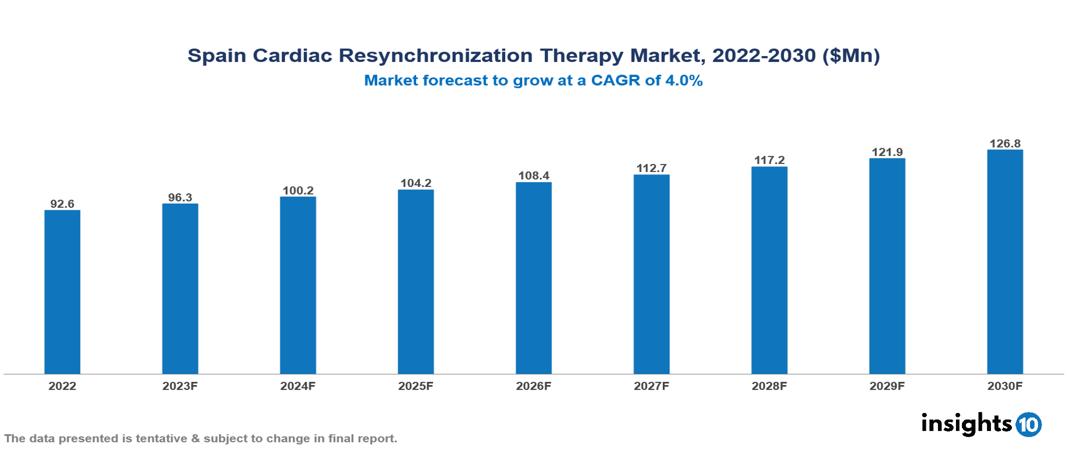
Spain Cardiac Resynchronization Therapy Market valued at $93 Mn in 2022, projected to reach $127 Mn by 2030 with a 4% CAGR. The worldwide increase in heart failure instances, propelled by aging demographics and cardiovascular diseases linked to lifestyle, stands as a pivotal factor fuelling the growth of the Cardiac Resynchronization Therapy (CRT) market. Presently, key players in this market encompass Abbott, Boston Scientific, Medtronic, Biotronik, Siemens Healthineers, St. Jude Medical, Sorin Group, Edwards Lifesciences, LivaNova, and Terumo.
Buy Now

Spain Cardiac Resynchronization Therapy Market Executive Summary
Spain Cardiac Resynchronization Therapy Market valued at $93 Mn in 2022, projected to reach $127 Mn by 2030 with a 4% CAGR.
Cardiac resynchronization therapy (CRT) is a medical procedure that involves the use of a pacemaker to correct the heart's rhythm through a minor surgical intervention. The CRT pacemaker, implanted beneath the skin, coordinates the timing between the upper and lower heart chambers and synchronizes the left and right sides of the heart. This therapy is particularly important for individuals with heart failure, as it helps to address inadequate pumping and fluid accumulation in the lungs and legs due to the asynchronous beating of the heart's lower chambers. In some cases of severe heart rhythm issues, an implantable cardioverter-defibrillator (ICD) may be combined with CRT therapy. The CRT device connects wires from the pacemaker to both sides of the heart, employing biventricular pacing to ensure coordinated contractions and optimize overall heart function.
In 2023, Spain faced a significant problem with heart attacks, prevalent in 2.4% of the population, leading to cardiovascular diseases being the leading cause of death with a mortality rate of 251.8 per 100,000 people. The lack of control over cardiovascular risk factors was the main reason for this issue. Ischemic heart disease, a common cause of heart attacks, affects 2.8% of the population. Comprehensive public health programs addressing lifestyle choices, genetics, and healthcare practices are urgently needed to reduce the impact of cardiovascular diseases in Spain. A holistic approach including preventive measures, early detection, and effective management is essential to improve overall cardiovascular health and lessen the burden on the population.
Abbott declared in June 2023 that a clinical trial in Spain has started to evaluate the safety and effectiveness of its most recent EnSite X EP system, which is intended for cardiac mapping and ablation treatments and may provide improvements in pacemaker implantation accuracy and efficiency. At the same time, Boston Scientific received regulatory approval in Europe in May 2023 for their ground-breaking EMBLEM MRITM pacemaker system, which became the first MRI-safe pacemaker authorized in the region.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Heart Failure: Due to demographic changes and rising rates of obesity and diabetes, heart failure is becoming more and more common in Spain, which is a serious health concern. Heart failure is becoming more and more common as the population ages, necessitating creative and practical treatment approaches. For heart failure, cardiac resynchronization therapy, or CRT, has shown to be a dependable and effective treatment. The increasing need for CRT underscores its significance as a critical intervention in the management of the rapidly spreading heart failure pandemic.
Growing Awareness and Education: Another factor contributing to the increasing demand for CRT is the growing awareness and education surrounding its benefits. Public awareness campaigns and educational initiatives targeting healthcare professionals have played a pivotal role in disseminating information about the efficacy of CRT. This heightened awareness is empowering individuals and healthcare providers to make more informed treatment decisions, thereby driving the demand for CRT.
Technological Advancements in CRT: Recent technological advancements in CRT devices have significantly transformed the landscape of heart failure treatment. The latest generation of CRT devices is distinguished by its compact design, enhanced energy efficiency, and sophisticated functionalities including wireless connectivity and remote monitoring. These improvements lead to better treatment outcomes in addition to increased patient comfort. The use of CRT therapy is being fuelled by its greater accessibility and appeal to patients and healthcare professionals.
Market Restraints
High Device Cost: Cardiac Resynchronization Therapy (CRT) devices come with a substantial financial burden, as their prices vary based on the brand and features. The cost is further escalated for MRI-compatible versions. The potential deterrents include limited insurance coverage and high out-of-pocket expenses, discouraging both patients and healthcare providers from choosing CRT therapy. In developing countries, the absence of government-imposed price caps on these devices exacerbates the financial barrier, limiting access to this advanced medical technology.
Stringent Regulatory Landscape: The introduction of new and innovative CRT technologies in the Spanish market is impeded by a stringent regulatory landscape governing medical device approval. Manufacturers face significant delays and increased costs while navigating these regulations, hindering the timely availability of CRT advancements. This regulatory burden poses a considerable challenge to market growth, as compliance becomes a time-consuming and costly aspect for manufacturers seeking to bring their products to the market.
Competition from Alternative Therapies: In addition to high costs and regulatory challenges, CRT faces competition from alternative therapies. Medications, lifestyle changes, His bundle pacing (HBP), left bundle branch area pacing (LBBAP), and leadless left ventricular (LV) pacing emerge as less expensive and invasive alternatives to CRT. While these alternatives may not directly replace CRT, they influence physician treatment decisions, particularly in specific patient segments. This competition introduces a dynamic element to the landscape, prompting healthcare professionals to carefully consider the most suitable and cost-effective treatment options for their patients.
Healthcare Policies and Regulatory Landscape
In Spain, the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS) is the regulatory body responsible for ensuring the effectiveness, safety, and quality of pharmaceuticals, including therapeutic drugs. The national healthcare system, which provides coverage to all citizens, plays a significant role in shaping healthcare policies. These policies are administered by the decentralized Systema Nacional de Salud (SNS) in Spain, where local regions manage healthcare services. Regulations at both the national and regional levels govern drug access, pricing, and reimbursement policies, with decisions often made through collaborative efforts.
Competitive Landscape
Key Players
- Abbott
- Boston Scientific
- Medtronic
- Biotronik
- Siemens Healthineers
- St. Jude Medical
- Sorin Group
- Edwards Lifesciences
- LivaNova
- Terumo
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Spain Cardiac Resynchronization Therapy Market Segmentation
By Product
- CRT-Defibrillator
- CRT-Pacemaker
By Age
- Below 44 years
- 45-64 years
- 65-84 years
- Above 85 years
By End-Users
- Hospitals
- Cardiac care Centres
- Ambulatory Surgical Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.