Spain Brain Cancer Therapeutics Market
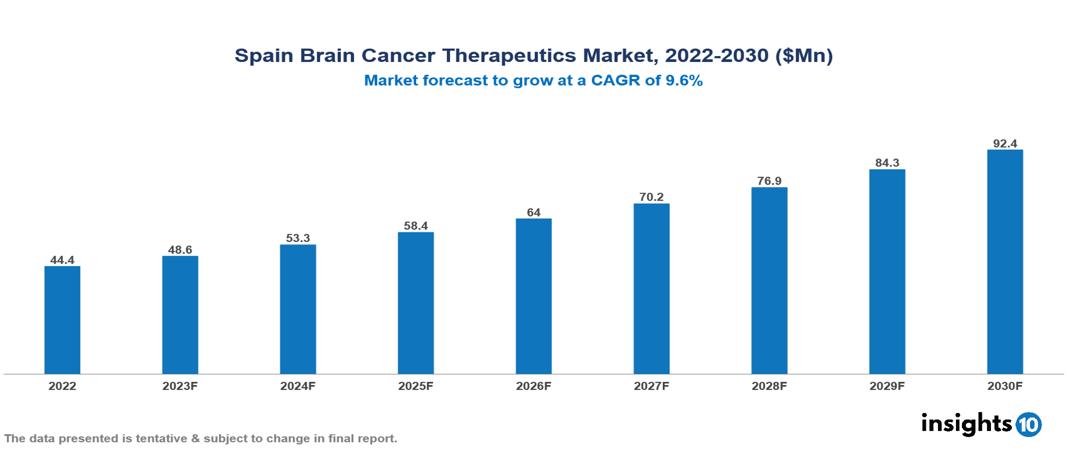
Spain Brain Cancer Therapeutics Market valued at $44 Mn in 2022, projected to reach $92 Mn by 2030 with a 9.6% CAGR. The expected rise in incidents of brain cancer, specifically glioblastoma multiforme, is projected to significantly drive the demand for brain cancer therapeutics, influencing the overall market scenario. The top leading pharmaceutical companies currently operating in the market are Roche, Merck & Co., Bristol Myers Squibb, Novartis, Pfizer, AstraZeneca, Eli Lilly, Sanofi, Takeda Pharmaceutical, and AbbVie.
Buy Now

Spain Brain Cancer Therapeutics Market Executive Summary
Spain Brain Cancer Therapeutics Market valued at $44 Mn in 2022, projected to reach $92 Mn by 2030 with a 9.6% CAGR.
The abnormal proliferation of brain cells serves as an indication of brain cancer, where these cells exhibit characteristics that can be either malignant (cancerous) or benign (non-cancerous). While the exact cause of brain cancer remains unclear, identified risk factors include exposure to ionizing radiation and a family history of brain tumors. Common symptoms associated with a brain tumor include headaches, gradual sensory decline, issues with balance, speech impediments, and hearing difficulties. The manifestation of these symptoms varies depending on factors such as the tumor’s size, location, and growth rate. Treatment approaches for brain cancer are determined by the tumor’s type, location, and size, with frequently utilized methods including radiosurgery, chemotherapy, radiotherapy, surgery, and the use of carmustine implants.
In Spain, brain tumors are relatively common, coming in at number 17 in incidence and number 11 in fatality. The projected number of new cases in 2020 was 4,420 across all age groups, including 1,133 cases in children (0–14 years old) and 564 cases in young people (15–39 years old). The predicted 5-year prevalence of all primary brain and central nervous system (CNS) cancers in Spain was 131,668 as of December 31, 2019. Meningioma was the most common kind among specialized types, it accounted for almost one-third of all cases and was benign in 99% of cases. Astrocytoma was a notable malignant brain tumor that mostly affected children, whereas glioblastoma, the most prevalent malignant brain tumor in adults, made up about 14.6% of all primary brain and CNS malignancies.
Glioblastoma is the most aggressive type of brain cancer. Researchers from the University of Lleida and the Institute for Research in Biomedicine of Lleida have discovered a novel cell death pathway in this disease. Mibefradil, a medication once used to treat hypertension, and its counterpart NNC-55-0396 were found to be effective in destroying glioblastoma cells and slowing the growth of tumors in mice. Remarkably, it was discovered that the anti-tumor action functions via another signaling mechanism, refuting earlier theories regarding T-channel blocking. This finding establishes the foundation for additional clinical trials, especially with NNC-55-0396 in glioblastoma patients, and highlights the possibility of repurposing currently available medications for the treatment of cancer. Simultaneously, Laminar Pharma, a biopharmaceutical pioneer in Spain, is committed to revolutionizing the treatment of brain cancer.
Market Dynamics
Market Growth Drivers
Increasing Incidence of Brain Cancer: Unfortunately, brain cancer is becoming more common in Spain, especially in the older population. Gliomas, the most common kind of brain tumor, are predicted to rise significantly by 2030. Spain is ranked 17th in the world for the incidence of brain tumors and 11th in terms of fatalities. The increasing incidence of brain cancer highlights the urgent need for efficient treatment alternatives, which in turn fuels the market's innovations to meet the nation's expanding needs for brain cancer-related healthcare.
Increasing Awareness and Advocacy: Public awareness of brain cancer is on the rise, thanks to media campaigns and advocacy initiatives. This heightened awareness contributes to early detection and a growing demand for diverse treatment options. Advocacy groups are actively striving to enhance treatment accessibility and tackle issues impacting both patients and their families.
Rising Demand for Personalized Medicine: A growing understanding of cancer genomes and tumor variety is fuelling interest in personalized medicine and providing the way to more specialized treatment approaches. With this strategy, specific mutations or pathways within patients' malignancies can be precisely targeted, potentially improving treatment outcomes. One of the main factors driving market expansion in Spain is the country's aggressive investment in customized medicine research and its application to clinical settings.
Market Restraints
Regulatory Hurdles: The rigorous regulatory demands and approval procedures may present obstacles in the development and introduction of novel drugs for brain cancer treatment, potentially causing delays in their market entry.
Limited Efficacy of Current Treatments: The effectiveness of current treatments for brain cancer might be constrained, emphasizing the necessity for groundbreaking innovations to markedly enhance patient outcomes. This constraint could potentially influence the market prospects of presently available drugs.
High Development Costs: The research and development costs associated with creating effective brain cancer treatment drugs can be substantial. Companies may face financial constraints in funding extensive clinical trials and obtaining necessary approvals.
Healthcare Policies and Regulatory Landscape
In Spain, the regulatory body in charge of guaranteeing the effectiveness, security, and caliber of pharmaceuticals, including treatment drugs, is the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS). The national healthcare system in Spain, which offers coverage to all citizens, has an impact on healthcare policies. Healthcare policies are governed by the decentralized Systema Nacional de Salud (SNS) in Spain, where healthcare services are managed by local regions. Drug access, pricing, and reimbursement policies are governed by regulations as well, and these decisions are frequently made at the national and regional levels.
Competitive Landscape
Key Players
- Roche
- Merck & Co.
- Bristol Myers Squibb
- Novartis
- Pfizer
- AstraZeneca
- Eli Lilly
- Sanofi
- Takeda Pharmaceutical
- AbbVie
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Spain Brain Cancer Therapeutics Market Segmentation
By Type
- Gliomas
- Meningiomas
- Pituitary Adenomas
- Vestibular Schwannomas
- Neuroectodermal Tumours
By Treatment
- Chemotherapy
- Immunotherapy
- Targeted Drug Therapy
- Radiation Therapy
- Others
By End-Users
- Hospitals
- Oncology Specialty Clinics
- Oncology Treatment Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.