Spain Biosensors Market Analysis
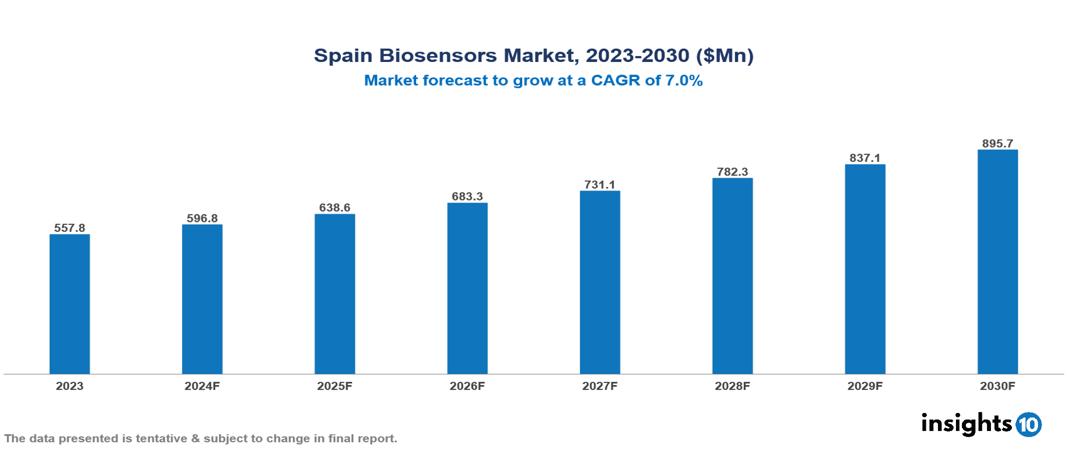
The Spain Biosensors Market was valued at $557.8 Mn in 2023 and is predicted to grow at a CAGR of 7.0% from 2023 to 2030, to $895.7 Mn by 2030. The key drivers of the market include increasing burden of chronic diseases, emphasis on preventative care, and technological advancements. The prominent players of the Spain Biosensors Market are Villard Medical, Bio-Rad International, Masimo Corporation, Meridian Bioscience, and Biosensors International, among others.
Buy Now

Spain Biosensors Market Executive Summary
The Spain Biosensors market is at around $557.8 Mn in 2023 and is projected to reach $895.7 Mn in 2030, exhibiting a CAGR of 7.0% during the forecast period.
Biosensors are analytical devices that detect changes in biological processes and transform the biological data into electrical signal. On the basis of sensor device and biological material, biosensors are classified into many types including electrochemical, thermal, optical, piezo-electric biosensors, and many more variants of these. Electrochemical biosensors work on the principle that many enzyme-catalyzed reactions consume or generate ions or electrons which causes change in electrical properties of the analyte being detected and can be used as a measuring parameter. Electrochemical biosensors are further subdivided into amperometric, potentiometric, impedimetric, and voltametric biosensors. One of the main applications of biosensors in this field is the detection of biomolecules which are either disease indicators or drug targets which aids in early disease diagnosis.
There is a significant burden on the healthcare sector of Spain due to the rising prevalence of chronic diseases such as diabetes, hypertension, and cancer, leading to increased mortality and morbidity in the country. The Spain Biosensors Market is thus driven by significant factors such as the increasing burden of chronic diseases, emphasis on preventative care, and technological advancements. However, stringent regulatory requirements, technical challenges, and data privacy and security issues restrict the growth and potential of the market.
The leading players of the Spain Biosensors Market are Villard Medical, Bio-Rad International, Masimo Corporation, Meridian Bioscience, and Biosensors International, among others.
Market Dynamics
Market Growth Drivers
Increasing Burden of Chronic Diseases: There is a significant burden on the healthcare sector of Spain due to the rising prevalence of chronic diseases such as diabetes, hypertension, and cancer. For instance, 10.3% of the population was suffering from diabetes as of 2021, according to the World Bank. Also, according to WHO, in 2022, the age standardized incidence cancer rates among men and women were 319.9 and 238.0, respectively, and 274.6 for both genders. Biosensors are very beneficial medical devices which help in the long-term monitoring of these chronic conditions which results in the potential growth of the Biosensors Market.
Emphasis on Preventative Care: Preventative care is becoming more important in healthcare as chronic diseases become more prevalent. Biosensors play a significant role in this by allowing individuals to monitor their health and detect early risk factors for chronic conditions. This early detection enables timely preventative measures, like lifestyle changes or medications, potentially delaying the development of chronic diseases. Thus, the emphasis on preventative care is driving the growth of the biosensors market.
Technological Advancements: Technological advancements in biosensors are continuously evolving. The novel use of fluorescence tagging and nanomaterials such as graphene and carbon nanotubes have led to increased biosensor sensitivity and improved detection limit. Next, the development of smaller biosensors has been made possible by developments in nanotechnology, materials science, and microfabrication techniques. These miniaturized devices are more effective for a range of applications due to greater sensitivity, specificity, and reaction times. Biocompatible materials are being used for wearable biosensors which minimizes the risk of rejection by the body and ensures patient comfort and compliance. Lastly, the integration of biosensors and AI and ML algorithms has made data analysis much more accurate and effective, which allows for personalized healthcare strategies and early disease detection.
Market Restraints
Stringent Regulatory Requirements: People’s health can be greatly impacted by biosensors, especially those meant for medical applications. Biosensor companies have to comply with the regulations of General Data Protection Regulation (GDPR) and Health Insurance Portability and Accountability Act (HIPAA). Rigorous testing is carried out by country-specific regulatory bodies of medical devices for accuracy, safety, and potential risks such as data security and biocompatibility. Although rigid guidelines ensure patient safety and market trust, the lengthy and expensive approval process can delay the launch of innovative biosensor technologies. This can ultimately prevent innovation and limit patient access to these tools. Also, strict regulatory requirements lead to higher costs to the developers which leads to an increased price of biosensors. Thus, strict regulatory requirements prevent the full expansion of the Biosensors Market.
Technical Challenges: For reliable and reproducible outcomes, maintaining long-term stability and shelf-life is essential. Factors like temperature variations, enzyme degradation, and calibration drift need to be managed. The development of miniaturized biosensors for wearables and point-of-care diagnostics is crucial, but integrating these into existing healthcare infrastructure is challenging and demands more work. Consequently, these technical issues are barriers to the expansion of the Biosensors Market.
Data Privacy and Security Concerns: Biosensor data contains extremely sensitive personal information about individuals, including genetic information and health parameters. Preventing breaches, unauthorized access, and misuse of data is crucial. Additionally, as biosensors get more networked, they are vulnerable to hacks that might alter data readings, interfere with sensor functionality, or steal private information. Additionally, data encryption is required to safeguard against unwanted access. Strong authentication and access control mechanisms must be implemented for this, which can be costly and challenging to administer. This has an impact on the biosensors market as a whole.
Regulatory Landscape and Reimbursement Scenario
The regulatory body for pharmaceuticals in Spain is the Spanish Agency of Medicines and Medical Devices (AEMPS) which operates under the Ministry of Health, Social Services, and Equality. AEMPS plays a critical role in safeguarding the public health in Spain by ensuring the efficacy, safety, and quality of pharmaceuticals, including both human and veterinary medications. For novel medications meant for the Spanish market, AEMPS evaluates applications and issues marketing authorizations. They also oversee the lifecycle of drugs that have previously received approval.
The pharmaceutical companies must submit a completed MAA (Marketing Authorization Application) which can be used for drugs meant for the Spain market (National Procedure) or for the drugs intended for commercialization throughout the European Union (EU) through the EMA (European Medicines Agency). Through the EMA, products can be authorized through the National Procedure, the Centralised Procedure (CP), Decentralised Procedure (DCP) or Mutual Recognition Procedure (MRP). In this case, AEMPS acts as a national competent authority (NCA) within the EMA framework. It then issues a final decision of either approval, conditional approval or refusal after conducting a review and evaluation of the MAA based on safety, efficacy, quality, and risk-benefit ratio. Even after entering the market, AEMPS and other stakeholders continue to monitor the drug’s safety profile through pharmacovigilance by gathering and examining adverse reaction data.
In Spain, the Ministry of Health (MOH) is the department of the central government responsible for approving reimbursement of medicinal products. When AEMPS grants final authorization for the packaging materials to be used in Spain, the process of pricing and reimbursement for a centrally approved pharmaceutical product starts. After AEMPS has given its approval for the product’s final packaging, it will document this decision and notify the MAH as well as the General Directorate for Pharmacy and Medical Devices, which is the body within the MOH competent to decide on reimbursement. The central government is the primary decision-maker in the reimbursement process. The MOH determines price and reimbursement after consulting with the ICPM and the General Directorate for Pharmacy and Medical Devices. The maximum amount that can be paid is determined by the ICPM after the General Directorate for Pharmacy and Medical Devices makes the initial decision about the product's reimbursement status.
Competitive Landscape
Key Players
Here are some of the major key players in the Spain Biosensors Market:
- Villard Medical
- Bio-Rad International
- Masimo Corporation
- Meridian Bioscience
- Biosensors International
- Nix Biosensors
- Abbott Laboratories
- Medtronic
- Siemens Healthcare
- F. Hoffmann-La Roche
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Spain Biosensors Market Segmentation
By Technology
- Electrochemical Biosensors
- Optical Biosensors
- Piezoelectric Biosensors
- Thermal Biosensors
- Nanomechanical Biosensors
By Product
- Wearable Biosensors
- Non-wearable Biosensors
By Application
- Medical Diagnostics
- Food Safety
- Environmental Monitoring
- Agriculture and Bioreactor Monitoring
- Other
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































