Spain Angelman Syndrome Therapeutics Market Analysis
Spain During the forecast period of 2023?2030, The Angelman Syndrome Therapeutics Market is anticipated to increase from $xx Mn in 2023 to $xx Mn by 2030, recording a CAGR of xx percent. An uncommon inherited neuro-developmental condition called Angelman Syndrome is marked by a severe developmental delay, sleep issues, jerky movements, and a lot of laughter. As the illness is more common, there are more government measures to manage it. This has also helped with research and development for condition treatment. These factors collectively represent the Angelman Syndrome Therapeutics Market's driving forces. Boston Children's Hospital, the Angelman Syndrome Foundation, Rady Children's Hospital in San Diego, General Hospital Corporation, Cincinnati Children's Hospital Medical Center, and others are major competitors in the market for treating Angelman syndrome.
Buy Now

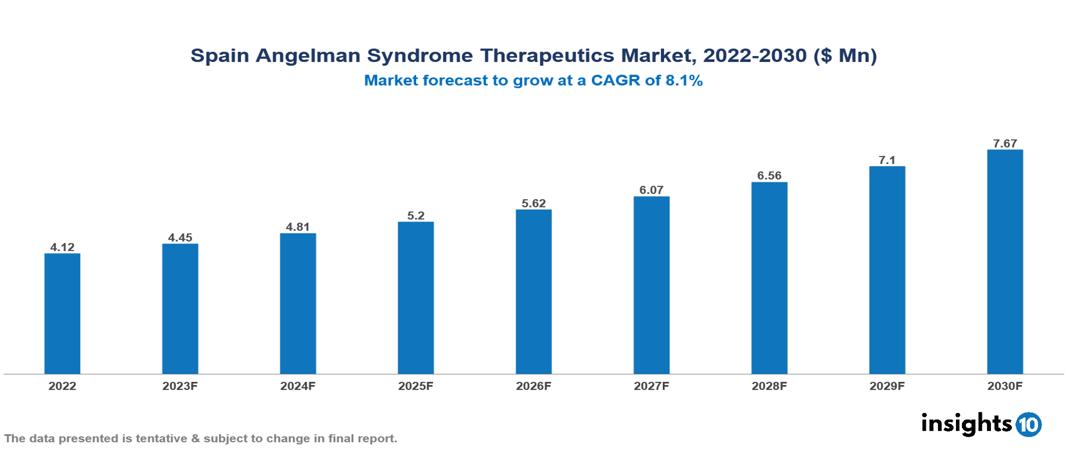
Spain Angelman Syndrome Therapeutics Market Analysis Summary
Spain Angelman Syndrome Therapeutics Market is valued at around $4.12 Mn in 2022 and is projected to reach $7.67 Mn by 2030, exhibiting a CAGR of 8.1% during the forecast period 2023-2030.
Angelman syndrome is a rare and complicated hereditary disorder that mostly affects the nervous system. Intellectual disability, issues with balance and movement, delayed development, and significant speech impairment are some of this health condition's most prevalent defining symptoms. Recurrent seizures and microcephaly, or small heads, can both occur in a number of affected children. AS is typically diagnosed between the ages of 3 and 7. The Angelman syndrome is expected to afflict more than 1 in 12 to 20 thousand people. There are numerous genetic alterations that cause AS, but they all result in a similar clinical presentation. A loss in the maternally contributed portion of chromosome 15q11–q13 accounts for about 70% of cases. DNA is methylation in the AS crucial region.
Typically, an AS diagnosis and test are performed after the infant is born. The majority of people with AS may be found via a blood test, which determines whether or not the UBE3A gene is operating normally. To identify the syndrome, fluorescent in situ hybridization (FISH) and CGH Array tests are used.
A recent study that disclosed new information about the disease process for Angelman syndrome (AS) found increases in autophagy, a method for removing cellular trash. These findings show that UBE3A deficiency enhances autophagy activity. One of the areas of the brain most affected by AS is the cerebellum. UX111, DTX401, DTX301, and UX701 are just a few of the gene therapy candidates in Ultragenyx's pipeline that have the potential to revolutionize the way many rare genetic illnesses are treated. Recently, the FDA authorized modifications to the GTX-102 Phase 1/2 trial in young patients with Angelman syndrome, enabling Ultragenyx to coordinate dosage scopes and speed up enrolment in the United States.
Boston Children's Hospital, the Angelman Syndrome Foundation, Rady Children's Hospital in San Diego, General Hospital Corporation, Cincinnati Children's Hospital Medical Center, and others are major competitors in the market for treating Angelman syndrome.
Market Dynamics
MarketDrivers
- Rising research and development spending
In the upcoming years, it is projected that the global market for treating Angelman syndrome would grow favourably as research and development spending increases to find better and more efficient treatments.
- Governmental programs
Rising government initiatives from nations across the world in an effort to create new and more effective treatment methods for Angelman syndrome are also projected to support the growth of the global market for treatments for Angelman syndrome in the upcoming years. The Global Angelman Syndrome Registry is an online database run by patient organizations that aim to compile data on the natural history of people with Angelman Syndrome in both children and adults.
- Strengthening Production and Development
Increasing research and development spending has led to the introduction of new technologies and the invention of products in the worldwide market for the treatment of Angelman syndrome.
- Creation of novel and specific
Researchers are currently working to make the treatment process more convenient by using effective treatment and technology.
Market Development
The prevalence of the syndrome has considerably increased around the globe. The first biobank in Australia with the aim of promoting research and treatment of rare genetic diseases caused by changes in genes on chromosome 15—including Prader-Willi Syndrome (PWS) and Angelman Syndrome (AS)—will be created at the Murdoch Children's Research Institute (MCRI).
RARE revealed that the FDA had authorized a protocol amendment after examining the phase I/II research of GTX-102 in young individuals with the uncommon neurogenetic disorder Angelman syndrome. The decision of this regulatory authority will let Ultragenyx to align dose levels in the United States with those being utilized in research cohorts outside of the United States.
"We have 500,000 children estimated to have Angelman syndrome in the globe, and we've identified under 10,000," claims Allyson Berent, chief science officer at the Foundation for Angelman Syndrome Therapeutics (FAST). We've started a number of newborn screening initiatives and a number of global diagnostic efforts for people who are not connected, underdiagnosed, or misdiagnosed in order to help fix that. In spite of the fact that there are currently no approved treatments for the uncommon neurogenetic disorder known as Angelman syndrome, Berent claims that his organization is advancing 13 potential treatments from mice to people, with a number of clinical trials already underway or scheduled to begin in the next 18 to 24 months.
Market Restraints
Children with Angelman syndrome may develop a wide range of neurological symptoms, all of varying degrees of severity. Ataxia, or the inability to coordinate voluntary movements, seizures, trouble sleeping, problems with movement and balance, a limited or impossible ability to converse, and others may also be present. Lack of understanding of this disease not only results in inaccurate diagnosis but also has an effect on the expansion of the global therapeutic market. The market for treatments for Angelman syndrome is also constrained by a paucity of data on the illness's prevalence and financial burden of care.
Key players
BioMarin Pharmaceutical uniQure Pfizer Alexion Pharmaceuticals Genzyme Sangamo Therapeutics Magenta Therapeutics Ceregene Intercept Pharmaceuticals Shire1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Spain Angelman Syndrome Therapeutics Market
By Diagnosis
- Neonatal detection of the disorder
- Blood test for UBE3A functionality
- Fluorescent in situ hybridization (FISH) test
- CGF Array Test
By Treatment
- Physical Therapy
- Communication Therapy
- Behaviour Therapy
- Others
By dosage form
- Oral
- Injectables
- Others
By Service Provider
- Hospitals
- Specialty Clinics
- Surgical Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































