Spain Actinic Keratosis Therapeutic Market Analysis
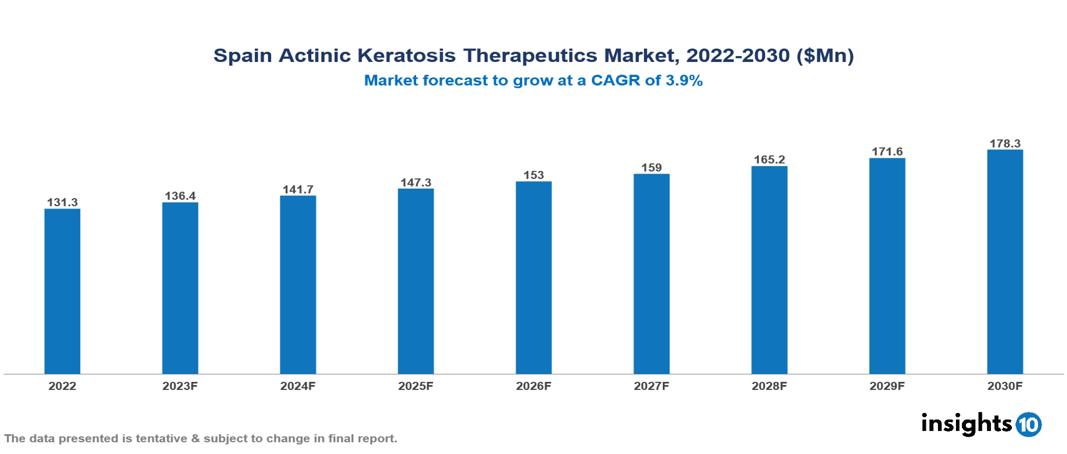
The Spain Actinic Keratosis Therapeutics Market was valued at $131 Mn in 2023 and is expected to reach $178 Mn by 2030, growing at a CAGR of 3.9%. The Spain Actinic Keratosis Therapeutics Market presents a promising outlook with rising prevalence, increasing public awareness campaigns to educate individuals about risks and the importance of early detection, increasing focus on early intervention, and development of novel treatment options. Major pharmaceutical companies like Bayer, Galderma, Pierre Fabre, Chemiplastics, Oncopharma, etc hold significant market shares
Buy Now

Spain Actinic Keratosis Therapeutic Market Executive Summary
The Spain Actinic Keratosis Therapeutic Market is at around $131 Mn in 2022 and is projected to reach US $178 Mn in 2030, exhibiting a CAGR of 3.9% during the forecast period
Actinic Keratosis (AK) is a prevalent skin condition that occurs when sun-damaged skin cells accumulate on sun-exposed areas. It is more prevalent in individuals with fair skin and can be triggered by prolonged sun exposure, leading to the risk of developing skin cancers like squamous cell carcinoma. Treatment options for AK include cryotherapy, which freezes affected skin to eliminate top layers, and curettage, which involves the scraping of affected skin. Surgical removal, laser resurfacing, and topical therapies like calcipotriol, fluorouracil, and tretinoin are also available. AK is commonly associated with prolonged sun exposure, particularly in individuals with fair skin, light eyes, and a history of extensive sun exposure or sunburn
AK affects around 25% of Spain's more than 45-year-old dermatology outpatient population; males and older age groups have the greatest incidence rates. The prevalence of AK varies throughout Spain. The northern, Mediterranean, and southern regions of Spain have the greatest point prevalence of AK. Since AK is underdiagnosed, early detection and treatment of these lesions require a proactive approach. The Spain Actinic Keratosis Therapeutics Market presents a promising outlook with rising prevalence, increasing public awareness campaigns to educate individuals about risks and the importance of early detection, increasing focus on early intervention, and development of novel treatment options
Major pharmaceutical companies like Bayer, Galderma, Pierre Fabre, Chemiplastics, Oncopharma, etc are gaining traction with newer options and treatment therapies
Market Dynamics
Market Growth Drivers
The Spanish market for AK therapeutics is expanding as a result of many reasons. Growing awareness and prevalence drive market expansion. The market is expanding as a result of rising AK prevalence and increased knowledge of the numerous dangers connected to sun exposure and healthy skin. The market is expected to increase significantly as a result of different strategic efforts implemented by industry competitors and rising awareness of AK. The market is expected to increase significantly as a result of different strategic efforts implemented by industry competitors and rising awareness of AK
Another key development factor in Spain is the availability of skilled healthcare professionals and advanced healthcare infrastructure. The availability of qualified healthcare personnel as well as improved healthcare infrastructure is expected to raise market revenue
The launch of novel treatments, the existence of major market competitors, and advantageous reimbursement policies are other important factors propelling market progress. Innovation and market expansion are encouraged by pharmaceutical firms' competition as well as their strategic alliances for the creation and promotion of AK medicines
Market Growth Restraints
While more recent therapies like imiquimod or photodynamic therapy may have restricted coverage or eligibility requirements, more traditional therapies like cryotherapy and topical fluorouracil are covered. This may impede the ability of new medications to reach patients and penetrate the market. Growth in the market may be impacted by Spain's convoluted and dynamic pharmaceutical pricing and reimbursement regulations. Regional differences in modern healthcare facilities, qualified healthcare personnel, and healthcare infrastructure may also have an impact on the market's growth
The population's cultural characteristics and beliefs may alter the patterns of adaptation for these more recent taxa. Certain patients can be deterred by the negative effects of a particular therapy or prefer non-invasive alternatives. Innovative but more expensive medications may not gain as much traction in the market as they could if healthcare providers and payers favour less expensive treatments like generic topical creams over more recent and pricey ones
While more recent therapies like imiquimod or photodynamic therapy may have restricted coverage or eligibility requirements, more traditional therapies like cryotherapy and topical fluorouracil are covered. These may impede the ability of new medications to reach patients and penetrate the market. It is projected that the availability of alternative natural remedies and their incorporation into insurance plans will impede market expansion, as some patients could choose to use this in lieu of traditional medical care
Healthcare Policies and Regulatory Landscape
Spain's healthcare policies and regulatory landscape are defined by a universal healthcare system based on universality, equity, and accessibility. The system is primarily supported by general taxes, guaranteeing that every person, regardless of work or financial condition, has access to medical care. The central state oversees the licensing and responsibility framework applicable to healthcare providers and professions in Spain, with implementation and enforcement assigned to the autonomous regions. These areas are in charge of developing particular rules and procedures for authorizing and enforcing appropriate healthcare legislation. The Law on Guarantees and Rational Use of Medicine and Healthcare Goods governs the regulation of medicine and healthcare goods in Spain, which harmonizes all relevant regulations. The Spanish Agency of Medicines and Medical Devices (AEMPS) is the therapeutics regulating authority in Spain. The AEMPS is in charge of evaluating and authorizing pharmaceuticals for human and veterinary use, as well as medical equipment. Furthermore, pharmaceutical pricing and reimbursement policies in Spain are intricate and vulnerable to change, which can have an influence on market growth
Notable Recent Updates
October 2023, Curativo approval (October 2023): Oncopharma's ingenol mebutate gel (Curativo) received marketing authorization in Spain,offering a first-line topical option for rapid treatment of moderate AK lesions on the face and scalp. This significantly expands treatment options for patients and could impact market share dynamics.
Competitive Landscape
Key Players
- Bayer
- Galderma
- Biofrontera
- Pierre Fabre
- Chemiplastics
- Oncopharma
- Aclaris Therapeutics
- Varens
- Hill Dermaceuticals
- LEO Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Spain Actinic Keratosis Market Segmentation
By Treatment Type
- Topical Treatment
- Procedural Modality
- Photodynamic Therapy
- Others
By Drug Class
- Nucleoside Metabolic Inhibitor
- Immune Response Modifiers
- NSAIDs
- Photo enhancer
- Other Drug Classes
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Online Providers
By Disease Type
- Clinical AK
- Subclinical AK
By End User
- Hospitals
- Private Dermatology Clinics
- Laser Therapy Centres
- Cancer Treatment Centres
- Spas and Rejuvenation Centres
- Homecare
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.