South Korea Radiotherapy Market Analysis
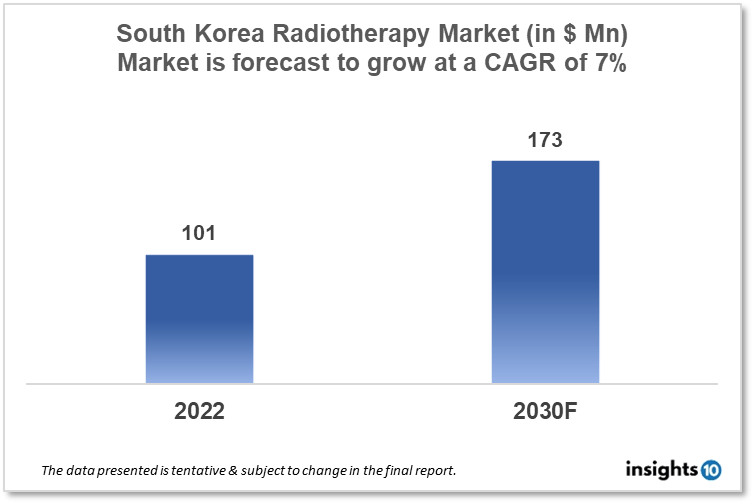
By 2030, it is anticipated that the South Korea Radiotherapy Market will reach a value of $173 Mn from $101 Mn in 2022, growing at a CAGR of 7% during 2022-30. The Radiotherapy Therapeutics Market in South Korea is dominated by a few domestic players such as HANRAY, Dongbang Medical, and Neusoft Medical Systems Korea. The radiotherapy market in South Korea is segmented into different types, technology, procedures, application, and end-user. The major risk factors associated with awareness of radiotherapy shortage of skilled staff, government initiatives and reimbursement policy. The demand for South Korea Radiotherapy is increasing on account of the rise in cancer cases in the country.
Buy Now

South Korea Radiotherapy Market Analysis Summary
By 2030, it is anticipated that the South Korean radiotherapy Market will reach a value of $173 Mn from $11 Mn in 2022, growing at a CAGR of 7% during 2022-30.
South Korea is without a doubt one of the most modern and industrialized countries in the world since it is home to multiple tech titans that drive advancement in a wide range of disciplines. Cancer incidence rates in South Korea remain slightly higher than the OECD average for both men and women. In 2010, there were 208,154 cancer patients in South Korea, which gradually climbed to 235,072 in 201.
There were 254,718 new cancer cases identified in 2019 and 81,203 cancer deaths reported. South Korea's total health expenditure will account for approximately 8.8% of the country's GDP in 2021. Global medical expenditure is predicted to increase by 7.5% over the previous year in 2021. Over 60% of the costs were covered by the government or the public health insurance system.
Market Dynamics
Market Growth Drivers Analysis
South Korea has among the best five-year relative survival rates in the world for some of the most common cancer types. South Korea is the Asia-Pacific region's third most active cancer trial site, accounting for 8.6% of all trials. From 2014 to 2019, the country ranked first among the most imaginative countries according to the Bloomberg Innovation Index. The Korea International Medical Association released a report saying that medical tourism has expanded as a result of the foreign patient legislation statute established in 2009. This regulation allows international patients to obtain longer-term medical visas and allows local facilities to offer medical tourism. These aspects could boost South Korea, Radiotherapy Market.
Market restrains.
In South Korea, systemic therapy is generally dependent on pharmacological approval from the Ministry of Food and Drug Safety (MFDS), but there is no similar provision for radiotherapy. The rate of RT use was 24.5% in 2010, and it climbed to 36.1% in 2019, which is not much in comparison to other countries, suggesting an oversupply scenario. These factors may deter new entrants into the South Korea Radiotherapy Market.
Competitive Landscape
Key Players
- Neusoft Medical Systems Korea- Neusoft Medical Systems Korea is a subsidiary of Neusoft Medical Systems, a Chinese medical equipment manufacturer. The company produces radiotherapy equipment such as linear accelerators and treatment planning systems
- Dongbang Medical- Dongbang Medical is a South Korean company that produces and distributes medical equipment, including radiation therapy equipment such as linear accelerators and CT simulators
- HANRAY- HANRAY is a South Korean company that specializes in radiation therapy equipment such as linear accelerators, CT simulators, and brachytherapy equipment
- Noninvasive Medical System- Noninvasive Medical System is a South Korean company that develops and manufactures radiation therapy equipment such as gamma knife systems and proton therapy systems
Notable Recent Deals
November 2022: TAE Life Sciences (TLS), a biological-targeted radiation treatment firm based in the United States, has announced a collaboration with HDX Corporation (HDX) to deliver TLS's targeted radiation therapy to South Korea. According to the terms of the agreement, HDX, a leading distributor of radiation therapy medical equipment and clinical services based in Seoul, will be the primary sales, marketing, service, and distribution channel in South Korea for the TLS Alphabeam System, which includes radiation oncology equipment, software, and boron target drugs.
Healthcare Policies and Reimbursement Scenarios
In South Korea, the regulations and reimbursement of radiotherapy are overseen by the Ministry of Health and Welfare (MOHW) and the National Health Insurance Service (NHIS). The MOHW is responsible for regulating the use of radiation in South Korea, including the use of radiotherapy. The Korean Society for Radiation Oncology (KOSRO) is the main professional organization for radiation oncologists in South Korea and works closely with the MOHW to develop guidelines and standards of practice for radiotherapy. The Korean Society for Radiation Oncology (KOSRO) is the main professional organization for radiation oncologists in South Korea and works closely with the MOHW to develop guidelines and standards of practice for radiotherapy.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Radiotherapy Segmentation
By Type (Revenue, USD Billion):
- External Beam Radiation Therapy
- Linear Accelerators
- Compact Advanced Radiotherapy Systems
- Cyberknife
- Gamma Knife
- ?Tomotherapy
- Proton Therapy
- Cyclotron
- ?Synchrotron
- Internal Beam Radiation Therapy
- Brachytherapy
- Seeds
- Applicators and Afterloaders
- Electronic Brachytherapy
- Systemic Radiation Therapy
- ?Others
?By Technology (Revenue, USD Billion):
- External Beam Radiotherapy
- Intensity-Modulated Radiation Therapy (IMRT)
- Image-Guided Radiation Therapy (IGRT)
- Stereotactic Radiation Therapy (SRT)
- 3D Conformal Radiation Therapy (3D-CRT)
- Particle Therapy
- Internal Beam Radiotherapy
- Brachytherapy
- High-Dose Rate Brachytherapy
- Low-Dose Rate Brachytherapy
- Image-Guided Brachytherapy
- Pulse-Dose Rate Brachytherapy
- Systemic Radiation Therapy
- Intravenous Radiotherapy
- Oral Radiotherapy?
By Application (Revenue, USD Billion):
- Breast Cancer
- Cervical Cancer
- Colon and rectum Cancers
- Stomach Cancer
- Lung Cancer
- Prostate Cancer
- Skin Cancer
- Liver Cancer
- Other types of cancer
By End User (Revenue, USD Billion):
- Hospitals
- Radiotherapy Centers & Ambulatory Surgery Centers
- ?Cancer Research Institutes
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































