South Korea Infectious Disease Therapeutics Market Analysis
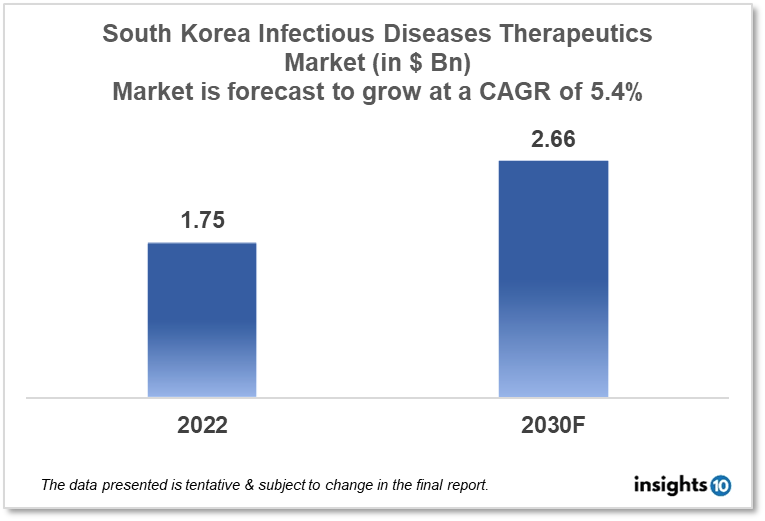
By 2030, it is anticipated that the South Korean infectious disease therapeutics market will reach a value of $2.66 Bn from $1.75 Bn in 2022, growing at a CAGR of 5.4% during 2022-2030. Infectious Disease Therapeutics in South Korea is dominated by a few domestic pharmaceutical companies such as Green Cross Corporation, SK Bioscience and GC Pharma. The Infectious Disease Therapeutics market in South Korea is segmented into different therapeutic areas and different diseases type. The major factors affecting the South Korean infectious disease therapeutics market are the increasing disease burden of communicable diseases like TB, hepatitis, and COVID-19 and the amount of healthcare funding for infectious disease treatment in various areas of South Korea.
Buy Now

South Korea Infectious Disease Therapeutics Analysis Summary
By 2030, it is anticipated that the South Korean infectious disease therapeutics market will reach a value of $2.66 Bn from $1.75 Bn in 2022, growing at a CAGR of 5.4% during 2022-2030.
South Korea is a high-income, developed country in Eastern Asia occupying the southern half of the Korean Peninsula. Infectious diseases are a major public health concern in South Korea, with diseases such as tuberculosis, hepatitis, and COVID-19 affecting a part of the population. The South Korean government has established a variety of programmes and regulations to promote access to infectious disease treatment, including free treatment for specific infectious diseases such as tuberculosis. In terms of pharmaceutical consumption, South Korea has a sizable and expanding pharmaceutical market.
According to a 2020 estimate, the South Korean pharmaceutical market was valued at over $20.2 Bn in 2020, with medications for infectious diseases being one of the most important therapeutic categories. Many medications used to treat infectious disorders, including antivirals and antibiotics, are available in generic form in South Korea. South Korea's government spends 8.4 % of its GDP on healthcare.
Market Dynamics
Market Growth Drivers Analysis
South Korea has seen several advances in infectious illness therapies in recent years. For example, with the release of new medications such as sofosbuvir and ledipasvir, the country has achieved great progress in the treatment of hepatitis C. South Korea has also been attempting to develop new diagnostic tools for infectious diseases, such as a COVID-19 quick diagnostic test. South Korea, being a developed country, has high private and state R&D spending, as well as a diverse industrial base. These aspects could boost the South Korean infectious disease therapeutics market.
Market Restraints
Developing new pharmaceuticals and treatments necessitates adherence to regulatory rules established by agencies such as South Korea's Ministry of Food and Drug Safety (MFDS). These requirements can be complicated and time-consuming, resulting in delays and higher expenses. South Korea has a great track record of enforcing intellectual property laws, although international enterprises may face difficulties in enforcing patents and other intellectual property rights. South Korea has a high youth unemployment rate, and the country's proximity to North Korea makes doing business challenging. These factors may deter new entrants into the South Korean infectious disease therapeutics market.
Competitive Landscape
Key Players
- Green Cross Corporation - Green Cross is a Korean biopharmaceutical company that develops and manufactures vaccines and therapeutics for infectious diseases such as influenza, hepatitis B, and COVID-19
- SK Bioscience - SK Bioscience is a Korean biopharmaceutical company that develops and produces vaccines and biologics for infectious diseases such as pneumonia, hepatitis B, and COVID-19
- GC Pharma - GC Pharma is a Korean biopharmaceutical company that develops and manufactures a range of therapeutics, including treatments for infectious diseases such as HIV/AIDS, hepatitis B and C, and influenza. The company has also been involved in the production of diagnostic tests and other medical products
- Korea Research Institute of Chemical Technology (KRICT)
- Vaxcell-Bio Therapeutics
Recent Notable Updates
January 2023: SK Bioscience, a global innovative vaccine and biotech company committed to promoting human health from prevention to cure around the world, introduced a new global partnership model at the Riyadh Global Medical Biotechnology Summit 2023 (RGBMS 2023) in the Kingdom of Saudi Arabia (KSA) as the first and only Korean biopharmaceutical company participating in the session.
February 2022: Curevo Vaccine (Curevo), a privately held clinical-stage biotechnology business dedicated to lowering the burden of infectious disease via the development of safe and highly effective vaccinations, announced the completion of a $60 Mn Series A fundraising transaction today. Janus Henderson Investors, EN Investment, and GC Pharma, a South Korean biopharmaceutical business focusing on the research and commercialization of vaccines, protein treatments, and therapeutic antibodies, were among the other investors.
Healthcare Policies and Reimbursement Scenarios
The Ministry of Food and Drug Safety regulates infectious illness medicines in South Korea (MFDS). The MFDS oversees assessing the safety, efficacy, and quality of all medications and medical devices, including those used to treat infectious diseases. South Korea has a national health insurance system that covers most of the population in terms of reimbursement. The National Health Insurance Service (NHIS) is in charge of handling the national health insurance programme, which covers a variety of healthcare services, including pharmaceuticals and infectious disease treatments.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Infectious Disease Therapeutics Segmentation
By Mode of Treatment (Revenue, USD Billion):
- Vaccines
- Drugs
By Applications (Revenue, USD Billion):
- HIV/AIDS
- Influenza
- Hepatitis
- Malaria
- Tuberculosis
- Others
By Disease Type (Revenue, USD Billion):
- Viral Diseases
- Bacterial Diseases
- Fungal Diseases
- Parasitic Diseases
- Others
By Target Organism (Revenue, USD Billion):
- Antibiotics
- Antivirals
- Antifungals
- Anti-Parasitic
- Others
By End User (Revenue, USD Billion):
- Hospitals and Clinics
- Ambulatory Care Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
By 2030, it is anticipated that the South Korean infectious disease therapeutics market will reach a value of $2.66 Bn from $1.75 Bn in 2022, growing at a CAGR of 5.4% during 2022-2030.
The Ministry of Food and Drug Safety regulates infectious illness medicines in South Korea (MFDS). The National Health Insurance Service (NHIS) is in charge of handling the national health insurance programme.
Infectious Disease Therapeutics in South Korea is dominated by domestic pharmaceutical companies such as Green Cross Corporation, SK Bioscience and GC Pharma.









































