South Africa Oncology Clinical Trials Market Analysis
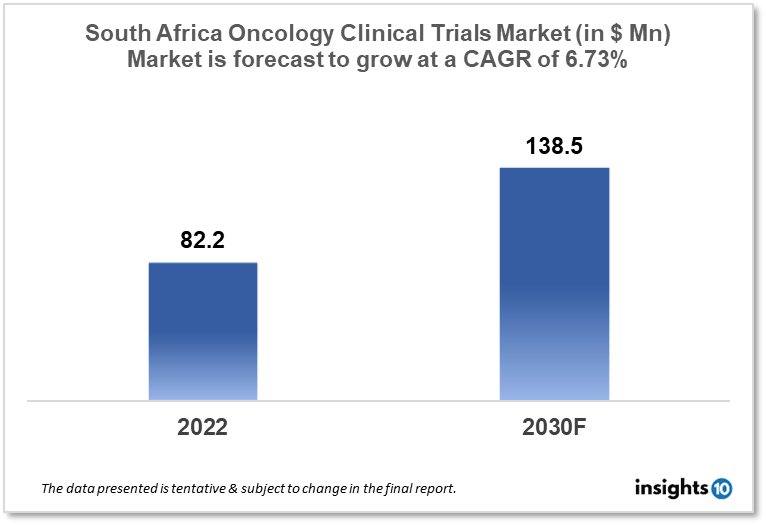
South Africa's oncology clinical trials market is projected to grow from $82.2 Mn in 2022 to $138.5 Mn by 2030, registering a CAGR of 6.73% during the forecast period of 2022-30. The market will be driven by the country’s rising cancer burden and regulatory framework for clinical trials. The market is segmented by phase, by study design & by indication. Some of the major players include Pfizer Inc., Roche Holding AG & Aspen Pharmacare.
Buy Now

South Africa Oncology Clinical Trials Market Executive Summary
South Africa's oncology clinical trials market is projected to grow from $82.2 Mn in 2022 to $138.5 Mn by 2030, registering a CAGR of 6.73% during the forecast period of 2022-30. South Africa's economy is the third biggest in Africa and the most industrialized, technologically proficient, and diverse on the continent. South Africa is one of just eight African nations with an upper-middle-income economy. The South African Health Products Regulatory Authority (SAHPRA) is the regulatory body in charge of pharmaceuticals, clinical research, medical devices, and radiation safety in South Africa. SAHPRA is responsible for clinical trial monitoring, authorization, and examinations in South Africa. All cancers are becoming more common, with the overall number of cases more than doubling between 2019 and 2030, from roughly 62,000 to 1,21,000 incident cases. Breast, cervical, basal cell carcinoma, squamous cell carcinoma, and colorectal cancer are the most often diagnosed malignancies in South African women, whereas prostate, basal cell carcinoma, squamous cell carcinoma, and colorectal, and lung cancer are the most commonly diagnosed cancers in men.
The broad and diversified patient population is one of the primary benefits of conducting oncology clinical trials in South Africa. The nation has a high cancer burden, and the frequency of various forms of cancer varies by area, making it a suitable site for research requiring a broad patient group. The nation is home to numerous world-class cancer treatment and research facilities, including the Chris Hani Baragwanath Academic Hospital, the Charlotte Maxeke Johannesburg Academic Hospital, and the Groote Schuur Hospital in Cape Town. South Africa has achieved significant success in the development of its medical research infrastructure, and it is en route to becoming a key participant in oncology clinical trials. If the government continues to invest in medical research, the number of trials will undoubtedly expand, and South Africa will play an increasingly vital role in the worldwide fight against cancer.
Market Dynamics
Market Growth Drivers
The broad and diversified patient population is one of the primary benefits of conducting oncology clinical trials in South Africa. The nation has a high cancer burden, and the frequency of various forms of cancer varies by area, making it a suitable site for research requiring a broad patient group. The availability of competent medical facilities and well-trained medical staff is another benefit of conducting oncology clinical trials in South Africa. The nation is home to numerous world-class cancer treatment and research facilities, including the Chris Hani Baragwanath Academic Hospital, the Charlotte Maxeke Johannesburg Academic Hospital, and the Groote Schuur Hospital in Cape Town. The regulatory framework for clinical trials in South Africa is also appealing to researchers. The government has created clinical research legislation that complies with international norms, making it an appealing venue for foreign pharmaceutical firms wishing to perform clinical trials.
Market Restraints
There are several challenges in conducting oncology clinical trials in South Africa. One of the most significant obstacles is a shortage of medical research funding, which might restrict the number of studies that can be undertaken. Also, there are some reservations regarding the possibility of ethical difficulties with informed consent, especially in rural communities where patients may not have access to the same degree of treatment or knowledge.
Competitive Landscape
Key Players
- Pfizer Inc.
- Roche Holding AG
- Novartis International AG
- AstraZeneca plc
- Merck & Co., Inc.
- Eli Lilly and Company
- Sanofi S.A.
- Bristol Myers Squibb Company
- Aspen Pharmacare (ZAF)
Notable Insights
December 2022: The Cancer Moonshot in a factsheet released additional steps to lower Africa’s cancer burden as part of the US - Africa leaders conference according to which:
- The National Cancer Institute (NCI) has committed $48.5 Mn to assist HIV-Associated Malignancy Research Centers, which include several universities and scientists from the United States and throughout the African continent, including South Africa
- The National Cancer Institute (NCI) has committed up to $60 Mn for the recently launched next phase of the Affordable Cancer Technologies (ACT) Program, which assists multidisciplinary research teams in Ethiopia, Kenya, Uganda, and South Africa in adapting, engineering, and implementing cost-effective new technologies for global cancer control
June 2022: Roche Pharma South Africa signed a Memorandum of Understanding (MOU) with Aspen, a worldwide speciality and branded multinational pharmaceutical firm.
February 2022: Debiopharm and Aspen partner to launch trelstar®, a prostate cancer drug, in South Africa
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Clinical Trials Regulation in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
6. Methodology and Scope
Oncology Clinical Trials Market Segmentation
By Phase (Revenue, USD Billion):
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design Outlook (Revenue, USD Billion):
- Epilepsy
- Parkinson's Disease (PD)
- Huntington's Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle regeneration
- Others
By Indication Outlook (Revenue, USD Billion):
- Interventional
- Observational
- Expanded Access
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































