South Africa Lymphoma Therapeutics Market Analysis
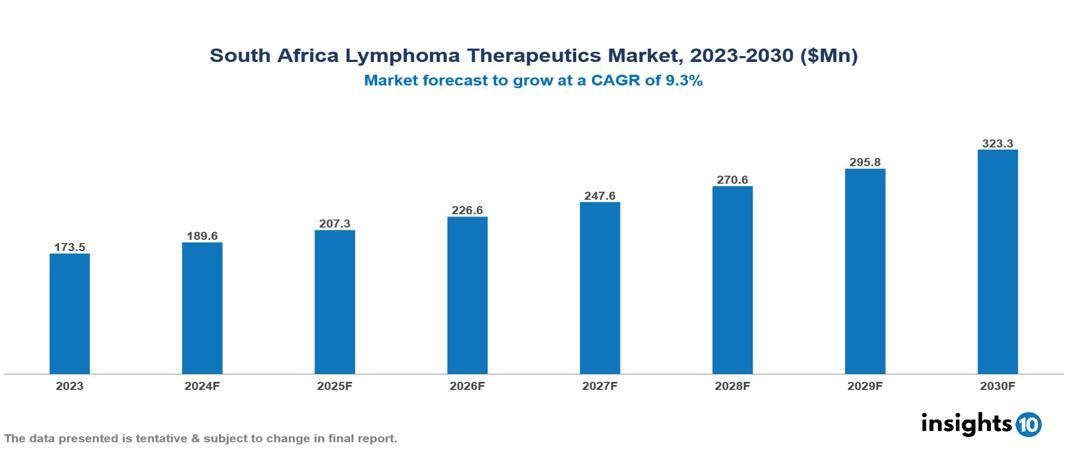
South Africa Lymphoma Therapeutics Market was valued at $173.50 Mn in 2023 and is predicted to grow at a CAGR of 9.30% from 2023 to 2030, to $323.33 Mn by 2030. The key drivers of this industry include increasing incidence, treatment advancements, and growing lymphoma awareness. The industry is primarily dominated by F. Hoffmann-La Roche Ltd, Celgene Corporation, Merck & Co, and Johnson & Johnson among others.
Buy Now

South Africa Lymphoma Therapeutic Market Executive Summary
South Africa Lymphoma Therapeutics Market was valued at $173.50 Mn in 2023 and is predicted to grow at a CAGR of 9.30% from 2023 to 2030, to $323.33 Mn by 2030.
Lymphoma, a group of cancers affecting the lymphatic system, consists of two primary types: Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). HL is identified by the presence of Reed-Sternberg cells, while NHL comprises multiple subtypes originating from lymphocytes. Common symptoms include swollen lymph nodes, fever, weight loss, fatigue, and itchiness. Treatment options, including chemotherapy, radiation therapy, immunotherapy, targeted therapy, and stem cell transplant, are tailored to the type, stage, and individual health condition. Consistent monitoring and follow-up care are essential for managing treatment outcomes and addressing potential side effects.
Non-Hodgkin Lymphoma (NHL) is a globally prevalent cancer, ranking among the top 10 for both men and women, with over 544,000 new cases in 2020. Incidence varies by region, with developed countries exhibiting higher rates due to factors like increased lifespan and improved detection methods. NHL's global incidence is projected to rise due to aging populations and other factors. Hodgkin Lymphoma (HL) is less common than NHL, with around 83,087 new cases in 2020. It typically affects younger adults and higher rates in developed countries. The Cancer Association of South Africa (CANSA) provides information on non-Hodgkin's Lymphoma, the most common type, stating it's the 4th most diagnosed cancer in men and 5th in women. The key drivers of this industry include increasing incidence, treatment advancements, and growing awareness of lymphoma. Restraints include healthcare accessibility challenges, affordability concerns, regulatory complexity, etc.
The industry is primarily dominated by F. Hoffmann-La Roche Ltd, Celgene Corporation, Merck & Co, and Johnson & Johnson among others.
Market Dynamics
Market Growth Drivers
Increasing Incidence: The rising prevalence of lymphoma in South Africa, fuelled by population growth, aging demographics, and environmental factors, drives demand for effective treatments, creating opportunities for market expansion.
Treatment Advancements: Ongoing innovations in lymphoma treatment, such as novel drugs, targeted therapies, and immunotherapies, offer patients more efficacious and tolerable options, stimulating market growth and improving patient outcomes.
Growing Awareness of Lymphoma: A heightened public awareness of lymphoma encourages early detection and intervention. As more individuals recognize the signs and symptoms of lymphoma, there's a greater likelihood of timely diagnosis and treatment, positively impacting market growth.
Market Restraints
Healthcare Accessibility Challenges: Socioeconomic disparities and geographic barriers impede access to specialized lymphoma treatment facilities and medications, particularly in rural and underserved regions, constraining market expansion.
Affordability Concerns: The high costs associated with lymphoma treatments, including newer and advanced therapies, pose financial obstacles for patients and healthcare systems in South Africa, limiting their adoption and utilization.
Regulatory Complexity: Complex and time-consuming regulatory processes, including drug approval and reimbursement requirements, may delay the introduction of new lymphoma treatments to the market, hindering market growth and innovation.
Regulatory Landscape and Reimbursement Scenario
In South Africa, pharmaceutical regulation for lymphoma treatment is overseen by two principal bodies. The South African Health Products Regulatory Authority (SAHPRA) is entrusted with the meticulous registration, evaluation, and monitoring of all health products, ensuring they adhere to rigorous safety, quality, and efficacy standards prior to distribution. Collaborating closely with SAHPRA, the South African Medicines Control Council (MCC) acts as an advisory entity, furnishing crucial scientific and technical counsel during the assessment of new medicines, thereby bolstering the integrity of the regulatory framework.
Regarding reimbursement for lymphoma treatment, South Africa operates through two main channels. The National Health Laboratory Service (NHLS) assumes a pivotal role in furnishing public healthcare laboratory services and actively participates in shaping treatment protocols for public healthcare facilities nationwide. Additionally, private medical schemes in South Africa offer a spectrum of coverage options for cancer treatment, inclusive of lymphoma. Patients are encouraged to meticulously review the details of their medical scheme plans to ascertain the scope of coverage available for their lymphoma treatment requirements, ensuring unfettered access to essential medical services.
Competitive Landscape
Key Players
Here are some of the major key players in the South Africa Lymphoma Therapeutic Market:
- F. Hoffmann-La Roche Ltd.
- Celgene Corporation (acquired by Bristol Myers Squibb)
- Merck & Co
- Johnson & Johnson
- AstraZeneca
- Novartis AG
- Gilead Sciences
- Pfizer Inc.
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
South Africa Lymphoma Therapeutic Market Segmentation
Type of Lymphoma
- B-Cell Lymphomas
- T-Cell Lymphomas
Treatment Type
- Radiation
- Chemotherapy
- Light Therapy
- Surgery
- Medication
- Others
Diagnosis
- Blood Cell Counts
- Tissue Biopsy
- Computed Tomography (CT) Scan
- Positron Emission Tomography (PET) Scan
- Magnetic Resonance Imaging (MRI) Scan
- Other
End-Users
- Hospitals
- Homecare
- Speciality Centres
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.