South Africa Kidney Cancer Therapeutics Market Analysis
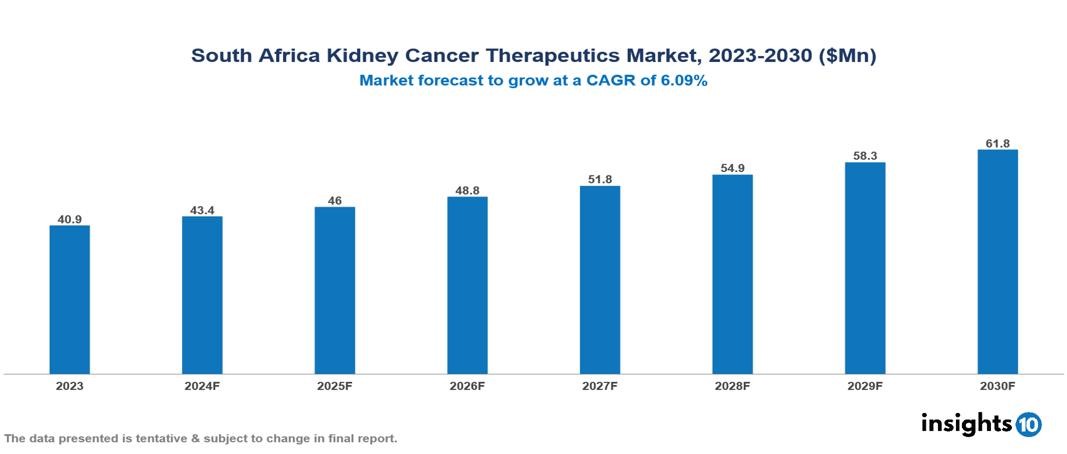
The South Africa Kidney Cancer Therapeutics Market was valued at $40.89 Mn in 2023 and is predicted to grow at a CAGR of 6.09% from 2023 to 2030, to $61.84 Mn by 2030. The key drivers of this industry include the increasing prevalence of kidney cancer due to lifestyle and environmental factors, early detection, and advancement in diagnostic tools. The key players in the industry are Pfizer, Novartis, Bayer, Roche, Bristol Myers Squibb, and AstraZeneca among others.
Buy Now

South Africa Kidney Cancer Therapeutics Market Executive Summary
The South Africa Kidney Cancer Therapeutics Market is at around $40.89 Mn in 2023 and is projected to reach $61.84 Mn in 2030, exhibiting a CAGR of 6.09% during the forecast period.
Kidney cancer also called renal cancer is a disease in which kidney cells become malignant (cancerous) and grow out of control, forming a tumor. The two most common types of kidney cancers are renal cell carcinoma (RCC), and Transitional cell carcinoma (TCC), accounting for the majority of the remainder. Other less common types of kidney cancers include Renal sarcoma and Wilms tumor. The causes of kidney cancer are not fully understood, although risk factors include smoking, obesity, high blood pressure, and a family history of the disease. Other symptoms may include unexpected weight loss, exhaustion, recurrent fever or night sweats, and anemia. Imaging studies such as CT scans or MRIs are often used to make a diagnosis, which is then confirmed with a biopsy. Treatment approaches depend on the stage and grade of the cancer. Common treatments include surgical removal of the tumor or entire kidney (nephrectomy), medication (including targeted therapy, which attacks cancer cells' specific weaknesses, and immunotherapy, which aids your immune system in fighting cancer), and ablation techniques, which use extreme heat, cold, or radio waves to destroy cancer cells.
Kidney cancer is the seventh most common neoplasm in men and the seventeenth most common in women in South Africa. It occurs more often in men than women. The market therefore is driven by significant factors like the increasing prevalence of kidney cancer due to lifestyle and environmental factors, supportive government initiatives, and improved diagnosis with better imaging techniques. However, high costs of treatment, stringent regulatory requirements, and side effects associated with the drugs restrict the growth of the market.
Leading players include Pfizer, known for its advancements in targeted therapies, and Bristol Myers Squibb, with a strong portfolio of immunotherapy drugs for advanced stages. Other contributors to the development of treatment for kidney cancer include Merck & Co., Roche, and Novartis.
Market Dynamics
Market Growth Drivers
Increasing Incidence and Prevalence: The rate of kidney cancer is rising universally, including in South Africa. There are 64,547 deaths annually due to cancer in South Africa, out of which Kidney cancer has a prevalence rate of 1.4%. Factors such as the aging population, lifestyle changes, and improved diagnostic techniques contribute to the increased detection of kidney cancer cases. Environmental exposures to toxins like herbicides, industrial chemicals, and air pollution are also emerging as potential contributors.
Technological advancements: Progress in drug development has helped with the introduction of targeted therapies, immunotherapies, and combination regimens for effective treatment. These innovative treatments offer several advantages over traditional chemotherapy. Targeted therapies and immunotherapies work by attacking cancer cells or the body's immune system, minimizing damage to healthy tissues, and reducing side effects. Combination regimens, where multiple drugs are used, can further enhance treatment efficacy.
Government initiatives: South Africa's National Cancer Control Policy Framework outlines a national strategy for addressing cancer prevention, diagnosis, treatment, and palliative care. The Department of Health works towards subsidizing the cost of some cancer drugs through the Essential Medicines List.
Market Restraints
High cost of treatment: Cancer drugs, particularly newer targeted therapies, and immunotherapies, come with a hefty price tag. These costs can place a significant financial burden on patients and healthcare systems. While these medications offer improved efficacy, the high cost can limit access for some patients. This can lead to difficult choices for individuals and families, potentially affecting treatment adherence and ultimately impacting treatment outcomes.
Regulatory Hurdles: The approval process is complex and it acts as a barrier to entry of new products. It can significantly delay approval for new kidney cancer medications and their availability to patients. This lag prevents patients from receiving these potentially life-saving treatments and poses a significant obstacle for them.
Side effects: Targeted therapies and immunotherapies, while offering significant advantages over traditional chemotherapy, are not without their drawbacks. These treatments can have significant adverse effects and safety concerns. Immunotherapy, on the other hand, may lead to immune-related toxicities, where the body's immune system attacks healthy tissues along with cancer cells. Careful patient monitoring and management of these side effects is essential to minimize their impact and ensure patient well-being.
Healthcare Infrastructure: South Africa still faces challenges in terms of access to quality healthcare facilities, especially in rural areas. Areas with limited access to these resources may face challenges in providing proper care. This scarcity hampers the ability to efficiently diagnose and treat patients, consequently affecting overall market growth.
Regulatory Landscape and Reimbursement Scenario
South African Health Products Regulatory Authority (SAHPRA) is responsible for evaluating, registering, and monitoring the safety and efficacy of all medicines, and medical devices. It emphasizes a "benefit-risk" framework when evaluating drugs, aiming to balance safety with potential patient benefits. SAHPRA is responsible for monitoring, evaluating, investigating, inspecting, and registering all health products with an additional responsibility of overseeing radiation control in South Africa. It works continuously to facilitate access to essential medicines for the South African population.
In South Africa, the pricing of pharmaceutical products, including cancer drugs, is regulated by the Pricing Committee of the Department of Health. The committee assesses the cost-effectiveness of drugs and may negotiate prices with manufacturers to ensure affordability and accessibility. South Africa has both public and private sources for financing the healthcare system. The government is in the process of implementing a National Health Insurance (NHI) system aimed at providing universal access to healthcare services. In the private sector, they have Fee-for-service (FFS) where healthcare providers bill the medical scheme directly for services rendered. The scheme reimburses the provider based on a pre-determined rate or tariff.
The process of obtaining a license for cancer drugs in South Africa involves the submission of detailed data on the drug's safety, efficacy, and quality. Once a registration application is submitted, SAHPRA conducts a thorough review of the data.
Competitive Landscape
Key Players
Here are some of the major key players in the South Africa Kidney Cancer Therapeutics Market:
- Novartis
- Pfizer
- Roche
- Bistrol Meyers Squibb
- Eisai
- Ipsen
- Dr. Reddy's Laboratories Ltd
- AstraZeneca
- Bayer
- Merck KGaA
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
South Africa Kidney Cancer Therapeutics Market Segmentation
By Cancer Type
- Clear cell RCC
- Papillary RCC
- Chromophobe RCC
- Transitional cell carcinoma
- Other Kidney cancers (Wilms tumor, Renal sarcoma, Collecting duct RCC)
By Treatment Type
- Targeted Therapy
- Immunotherapy
- Surgery
- Radiation Therapy
By Drug Classification
- Angiogenesis Inhibitors
- Monoclonal Antibodies
- mTOR Inhibitors
- Cytokine Immunotherapy (IL-2)
By Distribution Channel
- Hospitals
- Homecare
- Specialty Clinics
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.