South Africa Infectious Disease Drugs Market Analysis
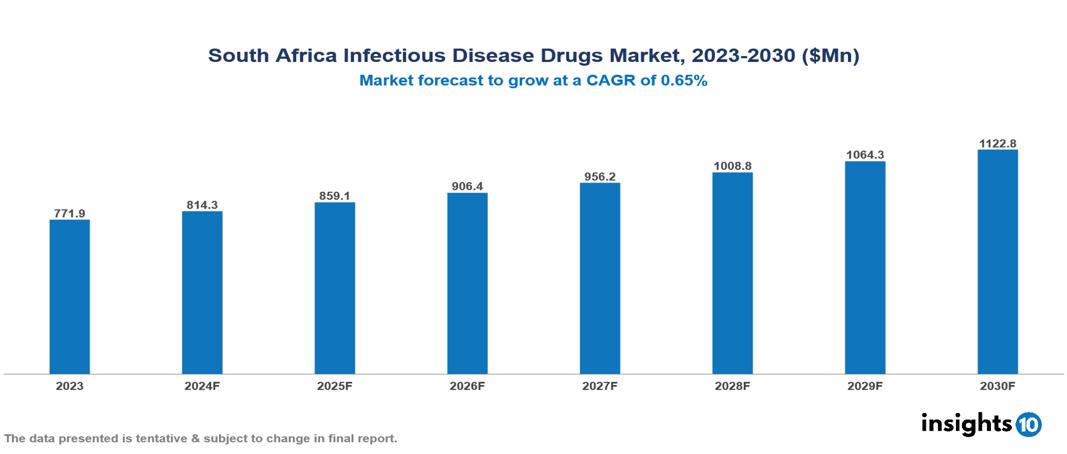
South Africa Infectious Disease Drugs Market valued at $0.77 Bn in 2023, projected to reach $1.12 Bn by 2030 with a 5.50% CAGR. The market is expanding due to factors such as high disease burden, government initiatives, and international partnerships and investments. The market is dominated by key players like Aspen Pharmacare, GlaxoSmithKline plc, Johnson & Johnson, Abbott Laboratories, Merck & Co., Mylan N.V., Cipla Limited, Roche Diagnostics, Sanofi, and Pfizer.
Buy Now

South Africa Infectious Disease Drugs Market Executive Summary
South Africa Infectious Disease Drugs Market valued at $0.77 Bn in 2023, projected to reach $1.12 Bn by 2030 with a 5.50% CAGR.
The Infectious Disease Drugs Market in South Africa develops, produces, distributes, and consumes drugs used to treat infectious diseases prevalent in the country. It covers a wide range of treatment areas, including antiretrovirals for HIV/ AIDS, antibiotics for bacterial infections, antimalarials, and antifungals, among others. Given South Africa's high prevalence of infectious diseases, the market is an important part of the country's healthcare infrastructure, influencing public health outcomes and policy decisions.
South Africa's Infectious Disease Drugs Market focuses on addressing common infectious diseases such as HIV/ AIDS, TB, malaria, and respiratory infections. It is fueled by government initiatives, rising healthcare spending, and a high prevalence of infectious diseases, making it an important part of the country's healthcare environment. Domestic and multinational pharmaceutical businesses are key players in the market, helping to enhance public health outcomes and lessen the burden of infectious diseases in South Africa.
In 2023, the projected value of the global drugs market for infectious diseases was $118.75 Bn. The rise has been linked to both an increase in diagnoses and government-sponsored public education campaigns about the diagnosis, treatment, and prevention of infectious diseases. A shifting market environment is indicated by the growing degree of generic competition. Long-term improvement requires removing financial barriers and increasing access to therapy, particularly in underdeveloped countries. More industrial research and development will result from this.
Pfizer has a significant presence in South Africa's HIV/ AIDS treatment landscape, providing medications such as Nevirapine and participating in partnerships or initiatives to increase ARV access. Pfizer manufactures vaccines for infectious diseases, including the pneumococcal conjugate vaccine (PCV) for pneumonia and meningitis and the rotavirus vaccine for rotavirus diarrhea.
Market Dynamics
Market Growth Drivers:
High Disease Burden: The high prevalence of infectious diseases like HIV/ AIDS, TB, malaria, and respiratory infections in South Africa leads to a demand for infectious disease medications and potential market expansion.
Government Initiatives: To battle infectious diseases, the South African government has put in place several initiatives and programs, including financing for efforts to prevent and treat the disease.
Foreign Investments and Partnerships: Knowledge sharing, technology transfer, and investment in the regional healthcare industry are made easier by partnerships between South African pharmaceutical companies, foreign pharmaceutical corporations, and international health organizations which promote the creation, manufacturing, and distribution of medications for infectious diseases, which propels the expansion of the market.
Market Restraints:
Healthcare Infrastructure Challenges: Problems with the healthcare workforce and infrastructure, such as subpar facilities and inadequate facilities, restrict access to medications for infectious diseases in some areas.
Limited Availability of Advanced Diagnostic Tools: Limited access to state-of-the-art diagnostic equipment and lab space, which causes delays in the diagnosis and start of therapy.
Regulatory Hurdles: Administrative processes and regulatory barriers impede the introduction and dissemination of novel treatments for infectious diseases.
Healthcare Policies and Regulatory Landscape
South African Health Products Regulatory Authority (SAHPRA) is the regulatory body in charge of approving pharmaceuticals in South Africa. The applicant company created the product dossier, which is regarded as a legal contract that must be approved by the Medicines Control Council (MCC). The medicine's Certificate of Registration, which doubles as a sales permission, attests to this. Any modifications the business makes after registering require MCC approval. Timelines may be further prolonged because SAHPRA has fewer resources than wealthier nations.
Competitive Landscape
Key Players:
- Aspen Pharmacare
- GlaxoSmithKline plc
- Johnson & Johnson
- Abbott Laboratories
- Merck & Co.
- Mylan N.V.
- Cipla Limited
- Roche Diagnostics
- Sanofi
- Pfizer
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Infectious Disease Drug Market Segmentation
By Disease
- HIV
- Influenza
- Hepatitis
- Tuberculosis
- Malaria
- Other
By Treatment
- Antibacterial
- Antiviral
- Antiparasitic
- Other
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































