South Africa Cardiac Pacemakers Market Analysis
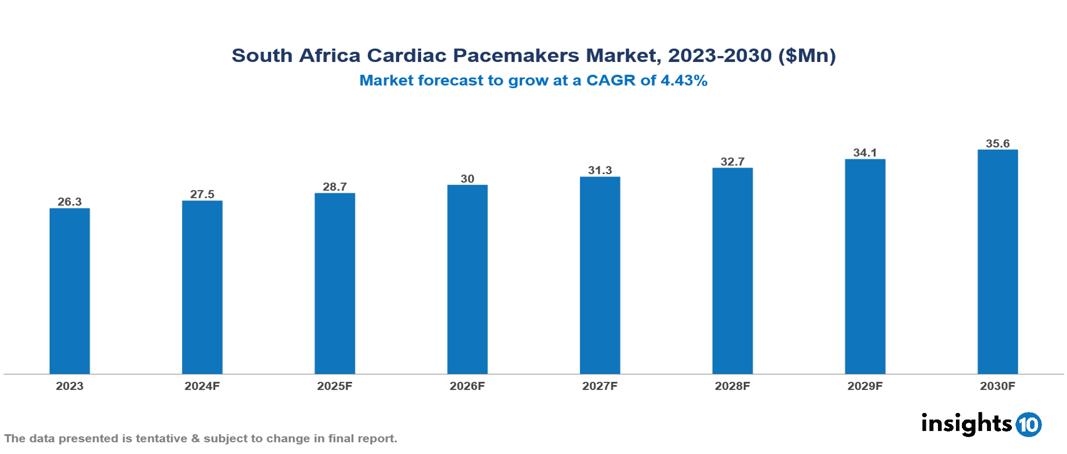
The South Africa Cardiac Pacemakers Market was valued at $26.30 Mn in 2023 and is predicted to grow at a CAGR of 4.43% from 2023 to 2030, to $35.63 Mn by 2030. The key drivers of this industry include the high prevalence of cardiovascular disease, the increasing aging population, and advancements in technology. The industry is primarily dominated by players such as Abbott Laboratories, Biotronik, Boston Scientific, and Medtronic among others.
Buy Now

South Africa Cardiac Pacemakers Market Executive Summary
The South Africa Cardiac Pacemakers Market was valued at $26.30 Mn in 2023 and is predicted to grow at a CAGR of 4.43% from 2023 to 2030, to $35.63 Mn by 2030.
A pacemaker is a small device designed to treat certain types of arrhythmias. Arrhythmias can cause the heart to beat too fast (tachycardia), too slow (bradycardia), or irregularly. When the heart beats too slowly, it can lead to inadequate oxygen supply to the body and brain, resulting in symptoms like lightheadedness, fatigue, fainting spells, and shortness of breath. Pacemakers send electrical pulses to maintain a normal heart rate and rhythm. Additionally, they can help synchronize the heart chambers, improving blood pumping efficiency, which is particularly beneficial for those with heart failure.
In South Africa, the national prevalence of stroke is reported at 4.29%. Modeling projections suggest a potential 48% increase for women and a 52% increase for men in the average number of premature deaths due to cardiovascular diseases (CVDs) by 2025 across Sub-Saharan Africa, assuming current risk factors persist. The market is driven by significant factors like the high prevalence of cardiovascular disease, the increasing aging population, and advancements in technology. However, high costs, limited healthcare access, and regulatory challenges restrict the growth and potential of the market.
Prominent players in this field include Abbott Laboratories, Biotronik, Boston Scientific, and Medtronic among others.
Market Dynamics
Market Growth Drivers
High Prevalence of Cardiovascular Disease: CVD is responsible for almost 1 in 6 deaths (17.3%) in South Africa. The increasing prevalence of heart conditions like stroke drives demand for advanced cardiac treatments such as pacemaker implants, making it a key market driver in South Africa's cardiac pacemaker market.
Increasing Aging Population: In 2022, seniors comprised 5.9% of South Africa's population. The United Nations forecasts that by 2030, South Africa will transition to an aging population category, with seniors making up 14% by 2060. This demographic shift drives the market for cardiac pacemakers in South Africa, as older adults are more susceptible to cardiovascular conditions, increasing the demand for pacemaker technologies to manage and treat these health issues effectively.
Advancement in Technology: Ongoing advancements in pacemaker technology, including enhanced battery longevity and advanced functionalities, appeal to both patients and healthcare providers, driving market expansion in the cardiac pacemaker sector in South Africa.
Market Restraints
High Cost: The high cost of pacemaker devices and procedures could restrict access for many patients in South Africa, potentially lowering demand within the cardiac pacemaker market.
Limited Healthcare Access: Unequal distribution of healthcare facilities and a scarcity of specialized cardiac care providers hinder access to pacemaker treatments, especially in rural areas, serving as a market restraint for the cardiac pacemaker market in South Africa.
Regulatory Challenges: Stringent regulatory requirements and prolonged approval processes for medical devices may prolong the introduction of new pacemaker technologies into the market, acting as a restraint for the cardiac pacemaker market in South Africa.
Regulatory Landscape and Reimbursement Scenario
Medical devices in South Africa, including pacemakers, are regulated by the South African Health Products Regulatory Authority (SAHPRA) under the Medicines and Related Substances Act of 2015, which includes regulations for both medical devices and in vitro diagnostic medical devices, as well as the Hazardous Substances Act. The regulatory framework uses a risk-based classification system ranging from Class A (low risk) to Class D (high risk), with pacemakers likely falling into higher-risk categories necessitating stringent approval processes. In terms of reimbursement, South Africa's healthcare landscape is evolving with the implementation of the National Health Insurance (NHI) system by the government. The NHI aims to improve access to high-quality healthcare services and achieve universal health coverage. Pacemakers are expected to be covered under the NHI package, which will provide a comprehensive set of defined health services.
Competitive Landscape
Key Players
Here are some of the major key players in the Cardiac Pacemaker Market:
- Abbott Laboratories
- Biotronik
- Boston Scientific
- Medtronic
- Siemens Healthineers
- LivaNova
- Cardinal Health
- MicroPort Scientific Corporation
- Edwards Lifesciences
- OSYPKA Medical
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
South Africa Cardiac Pacemakers Market Segmentation
By Product Type
- Single chamber
- Dual chamber
- Bi ventricular
By Application
- Bradycardia
- Tachycardia
- Other Arrhythmia
By End Users
- Hospitals
- Ambulatory Surgical Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































