South Africa Brugada Syndrome Market Analysis
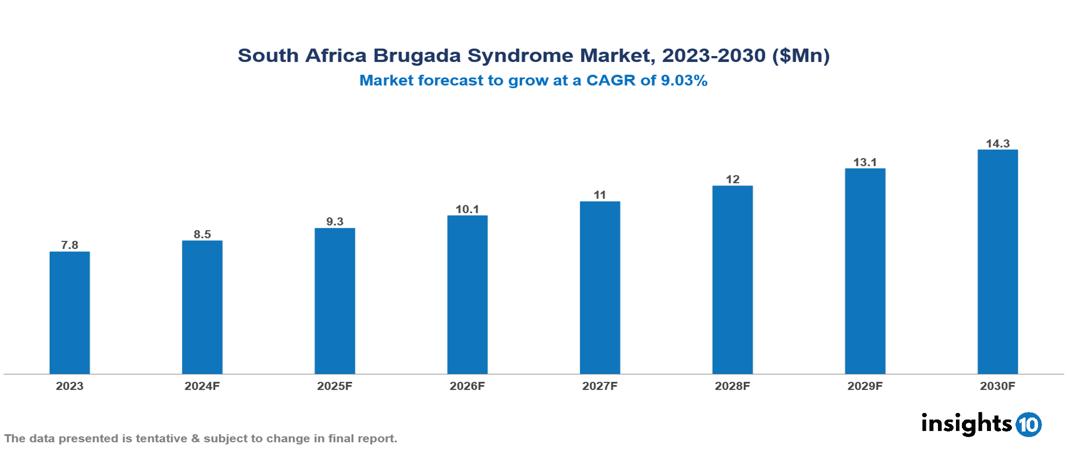
The South Africa Brugada Syndrome Market was valued at $7.8 Mn in 2023 and is predicted to grow at a CAGR of 9.03% from 2023 to 2030, to $14.3 Mn by 2030. The key drivers of the market include growing prevalence of cardiovascular diseases, aging population, and advancements in genetic testing. The prominent players of the South Africa Brugada Syndrome Market are Sun Pharma, Cipla, Bayer, Sanofi, Aspen Pharmacare, Astellas, and Medtronic, among others.
Buy Now

South Africa Brugada Syndrome Market Executive Summary
The South Africa Brugada Syndrome Market is at around $7.8 Mn in 2023 and is projected to reach $14.3 Mn in 2030, exhibiting a CAGR of 9.03% during the forecast period.
Brugada syndrome is a rare, but potentially threatening, genetic condition that causes abnormal electrical activity in the heart, leading to an increased risk of sudden cardiac death. People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart, i.e., the ventricles. Common signs and symptoms associated with Brugada Syndrome include dizziness, fainting, gasping and laboured breathing, particularly at night, irregular heartbeats or palpitations, extremely fast and chaotic heartbeat, and seizures. The risk factors for Brugada syndrome include family history of Brugada syndrome, being male, race, and fever.
In South Africa, CVD is the second leading cause of death after HIV/AIDS. The South Africa Brugada Syndrome Market is driven by significant factors such as growing prevalence of cardiovascular diseases, aging population, and advancements in genetic testing. However, high cost of treatment, side effects and complications of treatment, and limited R&D restrict the growth and potential of the market.
The major players of the South Africa Brugada Syndrome Market are Sun Pharma, Cipla, Bayer, Sanofi, Aspen Pharmacare, Astellas, and Medtronic, among others.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Cardiovascular Diseases: Non-communicable diseases (NCDs), including CVDs, are estimated to account for 43% of total adult deaths in South Africa. CVDs account for almost a fifth (18%) of these deaths. With the increasing commonality of cardiovascular conditions driven by aging populations, lifestyle changes, and higher incidences of hypertension and diabetes, there is a growing awareness and emphasis on genetic disorders like Brugada syndrome. The rising prevalence spurs more investment in diagnostic tools, research, and advanced treatments specifically for Brugada syndrome, as healthcare systems aim to tackle the wider range of cardiovascular health concerns. As a result, the demand for effective management and therapeutic options for Brugada syndrome is expected to increase, thereby driving market growth.
Aging Population: In 2022 the estimated population of South Africa included more than 5 Mn people aged 60 or older. The aging population is a crucial driver for the growth of the Brugada syndrome market. With the aging population, the incidence of cardiovascular diseases rises, intensifying the focus on related genetic conditions such as Brugada syndrome. Older adults are more likely to have regular cardiovascular screenings, resulting in higher detection rates of Brugada syndrome. This demographic trend boosts the demand for advanced diagnostic tools and effective treatments, thus promoting market growth for Brugada syndrome.
Advancements in Genetic Testing: Advancements in genetic testing are a key driver of the Brugada syndrome market by improving both the accuracy and accessibility of diagnoses. Enhanced genetic testing technologies enable more precise detection of the genetic mutations linked to Brugada syndrome, leading to earlier and more reliable diagnoses. As these technologies become more affordable and widely available, they boost diagnosis rates and support personalized treatment strategies, thus expanding the market for Brugada syndrome management and care.
Market Restraints
High Cost of Treatment: The high cost of treatment is a major constraint on the Brugada syndrome market, as it limits patient access and affordability. Advanced therapies, such as implantable cardioverter defibrillators (ICDs) and specialized medications, are often prohibitively expensive, creating financial barriers for many patients, especially in lower-income areas. This financial strain can restrict the use of essential treatments, impede market growth, and limit the overall availability of Brugada syndrome management solutions.
Side Effects and Complications of Treatments: Side effects and complications from treatments for Brugada syndrome, including implantable cardioverter defibrillators (ICDs) and specific medications can act as a restraint on market growth. Risks such as device malfunctions, infections, and psychological effects may discourage patients from choosing these interventions and complicate management approaches. These issues can reduce patient acceptance and adherence to treatment, thereby affecting the overall expansion and progress of the Brugada syndrome market.
Limited R&D: The limited R&D in Brugada syndrome significantly hinders market growth. Being a rare genetic disorder, Brugada syndrome attracts less attention and investment compared to more prevalent conditions, leading to slower progress in developing new treatments and therapies. This insufficient R&D restricts the creation of innovative diagnostic tools and effective treatments, impeding advancements in care options and reducing market opportunities for pharmaceutical and medical device companies.
Regulatory Landscape and Reimbursement Scenario
The regulatory body for pharmaceuticals in South Africa is the South African Health Products Regulatory Authority (SAHPRA), operating under the National Department of Health. SAHPRA ensures the safety, efficacy, and quality of all health products in South Africa, including human and veterinary medicines, medical devices, and complementary medicines.
Before new drugs can be sold in South Africa, SAHPRA evaluates applications and authorizes their commercialization. Additionally, they grant licenses to producers, distributors, and wholesalers of pharmaceuticals and medical devices. When evaluating novel drugs, SAHPRA examines and approves clinical trial applications to guarantee participant safety and ethical research procedures. Moreover, SAHPRA gathers and analyses information on adverse reactions reported by patients and healthcare professionals in order to actively monitor the safety of pharmaceuticals once they are placed on the market. Overall, the rigorous review process of SAHPRA helps ensure that only safe and effective medications reach the market, thus safeguarding the public health in South Africa.
South Africa's National Health Insurance (NHI) is a proposed reform aiming to transform the country’s healthcare financing system. Regardless of employment or income level, the NHI aims to provide all South African citizens and permanent residents with universal access to high-quality healthcare services. By establishing a unified funding source for the public and private healthcare sectors, the NHI seeks to overcome current disparities in healthcare access.
Competitive Landscape
Key Players
Here are some of the major key players in the South Africa Brugada Syndrome Market:
- Sun Pharma
- Cipla
- Bayer
- Sanofi
- Aspen Pharmacare
- Astellas
- Medtronic
- GE Healthcare
- Novartis
- Roche
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
South Africa Brugada Syndrome Market Segmentation
By Diagnosis
- Electrocardiogram
- Electrophysiology (Ep) Test
- Genetic Testing
By Treatment
- Implantable Cardioverter-Defibrillator
- Drug Therapy
By End User
- Hospitals
- Clinics
- Diagnostic Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































