Singapore Hypersomnia Therapeutics Market Analysis
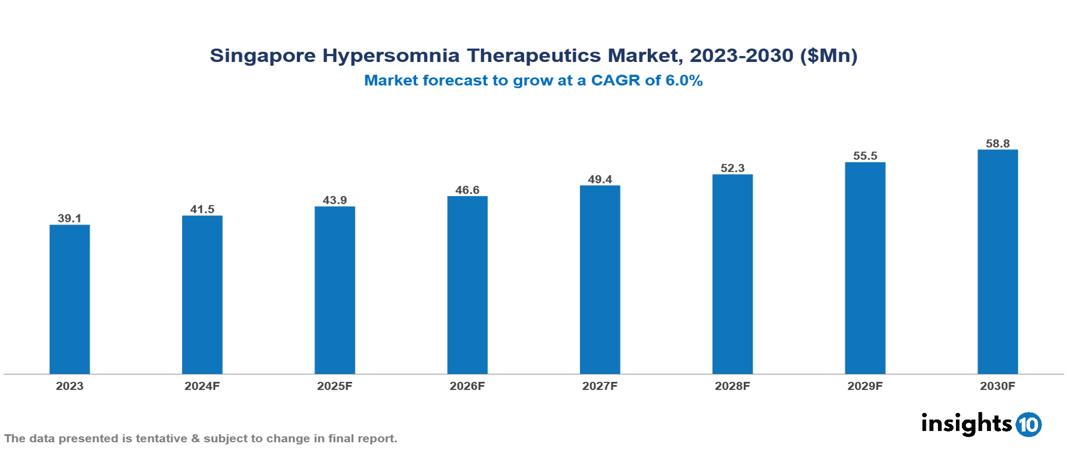
The Singapore Hypersomnia Therapeutics Market was valued at $39.10 Mn in 2023 and is predicted to grow at a CAGR of 6% from 2023 to 2030, to $58.79 Mn by 2030. The key drivers of this industry include the rising prevalence of sleep disorders, expanding geriatric population, early detection, and advancement in diagnostic tools. The key players in the industry are Pfizer, Sanofi, Sphaera Pharma, and Raffles Medical among others.
Buy Now

Singapore Hypersomnia Therapeutics Market Executive Summary
The Singapore Hypersomnia Therapeutics Market is at around $39.10 Mn in 2023 and is projected to reach $58.79 Mn in 2030, exhibiting a CAGR of 6% during the forecast period.
Hypersomnia is a group of neurological disorders characterized by excessive daytime sleepiness, despite getting enough sleep at night. This can significantly impair daily functioning and reduce quality of life. While the exact causes are still being researched, hypersomnia can be linked to underlying medical conditions, neurological disorders, or disruptions in sleep regulation mechanisms. People with hypersomnia often experience symptoms such as not feeling refreshed upon waking, feeling groggy and slow for extended periods after waking, and an excessive need for sleep despite sleeping for long periods. Chronic headaches, loss of appetite, excessive sweating, and depression have also been reported. Treatment for hypersomnia focuses on managing daytime sleepiness. This can involve adopting healthy lifestyle practices like eating well and exercising regularly. Medications like stimulants can also be prescribed to promote wakefulness. In some cases, cognitive behavioral therapy for insomnia (CBT-I) can help address underlying sleep habits that contribute to hypersomnia.
The prevalence of excessive daytime sleepiness, a key symptom of hypersomnia, is 9% in adults in Singapore. The market therefore is driven by significant factors like the increasing awareness about hypersomnia disorders, an aging demographic, and technological advancements. However, high costs of treatment, limited accessibility to specialized healthcare services, and strict regulatory approval impede market growth.
The leading pharmaceutical companies include Pfizer for the treatment of hypersomnia disorders in Singapore. Teva Pharmaceuticals, Raffle Medical, and Sanofi are also significant contributors to the hypersomnia therapeutics landscape, with continuous research and development activities.
Market Dynamics
Market Growth Drivers
Increasing Awareness about Hypersomnia Disorders: Increased public awareness campaigns and training of health professionals lead to more accurate diagnoses, increasing the potential patient base for these medications. Singapore Sleep Awareness Week (SSAW) is an annual event organized by the Singapore Sleep Society (SSS) to raise public awareness about sleep health and sleep disorders.
Expanding Geriatric Population: Hypersomnia can be more prevalent in older adults, due to changes in sleep architecture and underlying health conditions. The prevalence of hypersomnia disorders is expected to rise as the geriatric population accounts for 15% of the total population, driving demand for effective treatment options tailored to the unique needs of older adults.
Technological Advancements: Continuous technological innovations that improve the efficiency and effectiveness of Idiopathic Hypersomnia Treatment products and services are driving the market. Pharmaceutical companies are currently engaged in research and development to create improved medications for hypersomnia.
Market Restraints
High costs: The financial burden associated with the development and use of hypersomnia drugs is significant. This is particularly problematic in regions with limited healthcare coverage, as high costs restrict access to these therapies for patients, especially for long-term or chronic conditions
Strict Regulatory Approval Process: The approval of new drugs in Singapore involves a complicated procedure. Delayed market entry of more effective medications restricts patient treatment choices and impedes market growth.
Potential Side Effects: Hypersomnia medications, although effective in controlling severe sleepiness, frequently lead to several adverse reactions. Sadly, these reactions can discourage some people from pursuing therapy, or lead them to stop taking the medication before their full potential benefits are realized. As a result, this not only lowers the standard of living for those suffering from sleepiness disorders but also creates an obstacle to the expansion of the pharmaceutical market.
Regulatory Landscape and Reimbursement Scenario
The Health Science Authority (HSA) is responsible for drug approval in Singapore It operates under Ministry of Health. The HSA is responsible for regulating and approving health products, including therapeutic products, medical devices, cell, tissue, and gene therapy products ensuring they meet safety, quality, and efficacy standards.
Pharmaceutical companies submit a New Drug Application (NDA) to HSA. The NDA includes comprehensive data from preclinical and clinical studies, as well as information on the drug's formulation, manufacturing process, and proposed labeling. The HSA conducts a thorough review of the NDA. Following the registration of a product, pharmaceutical companies are obligated to fulfill a range of post-marketing responsibilities, including pharmacovigilance and variations reporting
Singapore has MediShield Life, a universal basic health care insurance that provides lifelong protection against large hospital bills and select outpatient treatments. They also have MediSave, a national medical savings scheme that helps cover out-of-pocket payments.
Competitive Landscape
Key Players
Here are some of the major key players in the Singapore Hypersomnia Therapeutics Market:
- Teva Pharmaceuticals
- Sphaera Pharma
- Jazz Pharmaceuticals
- Pfizer
- ITS Science & Medical
- Merck
- Sanofi
- Raffles Medical
- Fisher and Paykel Healthcare
- GlaxoSmithKline
- Theranexus
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Singapore Hypersomnia Therapeutics Market Segmentation
By Application
- Idiopathic Hypersomnia
- Narcolepsy Type-1
- Narcolepsy Type-2
By Product
- Anti-depressants
- Stimulants
- Sodium oxybate
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































