Singapore Brugada Syndrome Market Analysis
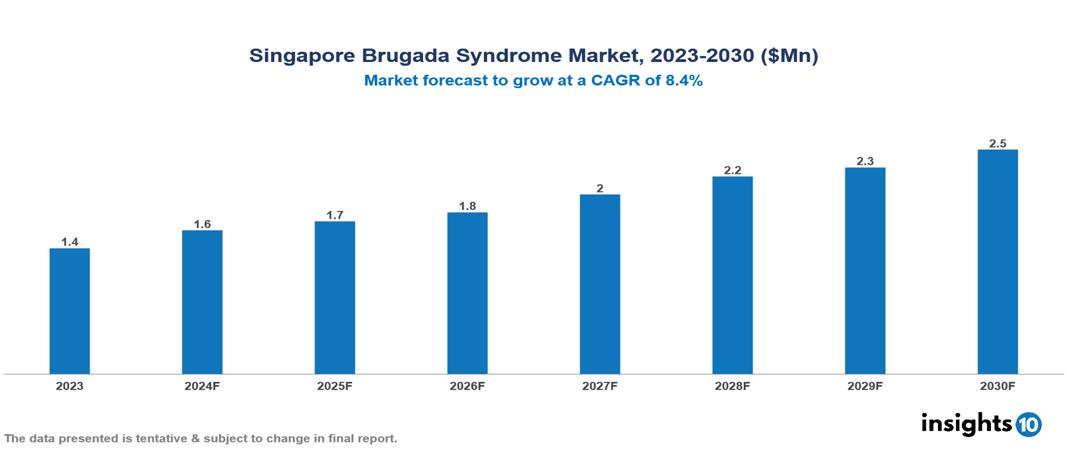
The Singapore Brugada Syndrome Market was valued at $1.4 Mn in 2023 and is predicted to grow at a CAGR of 8.4% from 2023 to 2030, to $2.5 Mn by 2030. The key drivers of the market include growing prevalence of cardiovascular diseases, government fundings, and advancements in genetic testing. The prominent players of the Singapore Brugada Syndrome Market are Bayer, Roche, Siemens Healthineers, Sanofi, Thermo Fisher Scientific, and Novartis, among others.
Buy Now

Singapore Brugada Syndrome Market Executive Summary
The Singapore Brugada Syndrome Market is at around $1.4 Mn in 2023 and is projected to reach $2.5 Mn in 2030, exhibiting a CAGR of 8.4% during the forecast period.
Brugada syndrome is a rare, but potentially threatening, genetic condition that causes abnormal electrical activity in the heart, leading to an increased risk of sudden cardiac death. People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart, i.e., the ventricles. Common signs and symptoms associated with Brugada Syndrome include dizziness, fainting, gasping and laboured breathing, particularly at night, irregular heartbeats or palpitations, extremely fast and chaotic heartbeat, and seizures. The risk factors for Brugada syndrome include family history of Brugada syndrome, being male, race, and fever.
In Singapore, cardiovascular diseases accounted for 31.4% of all deaths in 2022. The Singapore Brugada Syndrome Market is driven by significant factors such as growing prevalence of cardiovascular diseases, government fundings, and advancements in genetic testing. However, high diagnostic cost, regulatory approval challenges, and low patient population restrict the growth and potential of the market.
The major players of the Singapore Brugada Syndrome Market are Bayer, Roche, Siemens Healthineers, Sanofi, Thermo Fisher Scientific, and Novartis, among others.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Cardiovascular Diseases: In Singapore, cardiovascular diseases, including heart disease and stroke, claim the lives of 23 people daily, accounting for 31.4% of all deaths in 2022. This indicates that nearly one in three deaths is attributed to these conditions. As cardiovascular issues become more common, there is growing focus and investment in diagnostic and treatment solutions for related disorders like Brugada syndrome. This increased awareness and demand for specialized care contribute to the expansion of the market, fostering advancements in the detection, management, and treatment of Brugada syndrome in the region.
Government Fundings: The “Rare Disease Fund” of Singapore contributes to the growth of the Brugada syndrome market by offering financial support for the research, diagnosis, and treatment of rare conditions. By directing resources towards rare diseases like Brugada syndrome, the fund helps offset the expenses of advanced genetic testing, cutting-edge treatments, and specialized medical care. This funding fosters the development of new therapies and improves access to essential medical services for Brugada syndrome patients, thereby driving market expansion and enhancing patient outcomes.
Advancements in Genetic Testing: In Singapore, advancements in genetic testing are crucial for driving the growth of the Brugada syndrome market by enhancing the precision and speed of identifying individuals at risk. These improved technologies enable more accurate diagnoses, which facilitate early intervention and personalized treatment approaches. As diagnostic capabilities improve, patient awareness rises, leading to a higher demand for targeted therapies. This, in turn, accelerates market expansion and promotes innovation in managing Brugada syndrome.
Market Restraints
High Diagnostic Costs: Despite the government funding, the high cost of advanced diagnostic tests and genetic screening for rare heart conditions such as Brugada syndrome can be excessively expensive. This includes the expenses related to diagnostic procedures, advanced genetic testing, and ongoing treatments such as implantable cardioverter-defibrillators (ICDs). Thus, cost restricts accessibility for many patients and hinders market growth.
Regulatory Approval Challenges: Regulatory approval challenges for rare disease treatments in Singapore pose significant constraints on the Brugada syndrome market. The approval process for new therapies and diagnostic tools is often lengthy and intricate, requiring extensive evaluation by the Health Sciences Authority (HSA) to ensure safety and efficacy. For rare conditions like Brugada syndrome, the small patient population and specialized nature of these treatments can lead to increased scrutiny and higher barriers to entry. This can delay the introduction of innovative treatments and diagnostics, affecting overall market growth.
Low Patient Population: The small patient population with Brugada syndrome in Singapore makes it a niche market. With only a limited number of affected individuals, there is diminished demand for specialized diagnostic tools and treatments, which in turn deters investment from pharmaceutical companies and researchers. This limited patient base can result in lower revenue potential for companies targeting Brugada syndrome, reducing their motivation to develop and market new therapies. As a result, the market's growth is restricted by the lack of economic incentive to invest in advancements for this rare condition.
Regulatory Landscape and Reimbursement Scenario
The Health Sciences Authority (HSA) is the board under the Ministry of Health (MoH) of Government of Singapore which is responsible for ensuring the safety, efficacy, and quality of pharmaceuticals and medical devices, thus safeguarding the public health. The HSA conducts a thorough evaluation for applications from pharmaceutical companies seeking to register and market new drugs in Singapore.
The HAS’s responsibility extends beyond just granting marketing authorizations, and includes collecting and analysing data on adverse drug reactions reported by the patients. Also, the HAS safeguards the participants of the clinical trial by ensuring their ethical principles and safety above all. Overall, the HAS plays a crucial role in protecting patient health, ensuring quality, and promoting innovation.
The reimbursement scenario in Singapore’s healthcare system is aimed at achieving affordability and access to quality care for all patients. Regarding the public healthcare system, Singapore has a two-tiered healthcare system that consists of a subsidized public system (MediShield Life and MediSave) and a private system. The MediShield Life is an obligatory national health insurance program that covers the basics of hospital expenses in certain private hospitals with Class B2 and C ward accommodations as well as authorized public hospitals. MediSave is a mandated savings program where people save funds for their future medical expenses. With specific restrictions, it can be used in both public and private hospitals for both inpatient and outpatient care, including the certain medicines. The Integrated Shield Plans (IPs) are voluntary private health insurance plans that complement MediShield Life by providing broader coverage, including higher hospitalization reimbursements, coverage for outpatient care and medications, and coverage in private hospitals with higher-class wards.
Competitive Landscape
Key Players
Here are some of the major key players in the Singapore Brugada Syndrome Market:
- Bayer
- Roche
- Siemens Healthineers
- Sanofi
- Thermo Fisher Scientific
- Novartis
- Boehringer Ingelheim
- Merck
- Johnson & Johnson
- B. Braun
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Singapore Brugada Syndrome Market Segmentation
By Diagnosis
- Electrocardiogram
- Electrophysiology (Ep) Test
- Genetic Testing
By Treatment
- Implantable Cardioverter-Defibrillator
- Drug Therapy
By End User
- Hospitals
- Clinics
- Diagnostic Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































