Singapore Blood Disorder Therapeutics Market
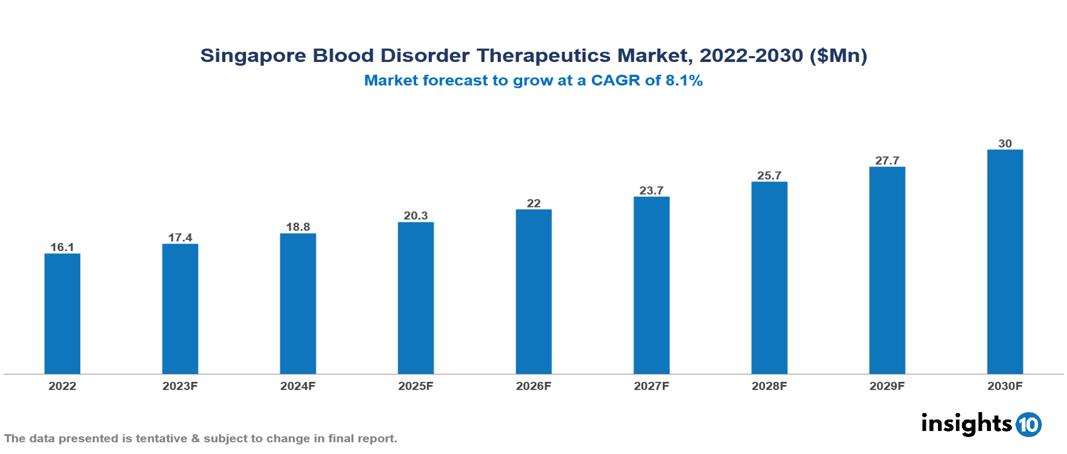
Singapore Blood Disorder Therapeutics Market valued at $16 Mn in 2022, projected to reach $30 Mn by 2030 with a 8.1% CAGR. The market is expanding due to the rising prevalence of blood disorders brought on by aging populations and unhealthy lifestyle choices, therapeutic advancements supported by technology developments, and government programs that fund research and provide affordable therapies. The Singapore Blood Disorder Therapeutics Market encompasses various players across different segments, including Takeda, Sanofi, Novo Nordisk, CSL Ltd, Pfizer, Bayer, BioLife Sciences, Agenus, Spark Therapeutics, Abeo Therapeutics, etc, among various others.
Buy Now

Singapore Blood Disorder Therapeutics Market Executive Summary
Singapore Blood Disorder Therapeutics Market valued at $16 Mn in 2022, projected to reach $30 Mn by 2030 with a 8.1% CAGR.
A blood disorder is a medical ailment when there is an issue with your white blood cells (which fight infection), red blood cells (which carry oxygen throughout your body), or platelets—small circulating cells that are essential for the development of clots. Blood diseases are ailments that affect the proper functioning of the blood. Depending on the type of blood condition, symptoms vary. But there are other typical signs like weight loss and inexplicable exhaustion. The majority of blood illnesses either reduce the quantity of nutrients, proteins, platelets, or cells in the blood or affect how well they operate. Most blood problems may be handed down through families and are caused by mutations in certain gene regions. These may or may not be malignant, depending on the underlying causes. Blood problems can also arise as a result of certain medical illnesses, drugs, and lifestyle choices. For patients with a platelet problem, pharmacotherapy choices include drugs like romiplostim, which stimulates the bone marrow to create more platelets. Antibiotics can aid in the battle against infections in cases with white blood cell abnormalities. Supplements containing iron and vitamins B-9 or B-12 can be used to treat deficiency-related anemia. Blood diffusion therapy, chemotherapy, radiation therapy, immunotherapy, stem cell transplantation, and other treatments are available for more severe disorders.
In Singapore, the frequency of blood disorders varies depending on the demographics. Adult cases of blood cancer have increased recently. In Singapore, there are thought to be 10–12 instances of hemophilia for every 100,000 men. The prevalence of hemophilia A is five times higher than that of hemophilia B.
The market is expanding due to the rising prevalence of blood disorders brought on by aging populations and unhealthy lifestyle choices, therapeutic advancements supported by technology developments, and government programs that fund research and provide affordable therapies.
Almost every pharma player in the Singapore market is involved in the research and development of novel pharmaceuticals. Takeda and CSL Ltd. are significant players in the plasma-derived therapeutics market. Strong positions in recombinant factor VIII medicines for hemophilia are held by Novo Nordisk and Bayer. Important blood cancer medicines are provided by Amgen and AstraZeneca. Leading local player BioLife Sciences specializes in cutting-edge blood-based treatments.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Blood illnesses: Blood illnesses such as hemophilia, anemia, and blood malignancies are becoming more common as a result of aging populations and poor lifestyle decisions. The market is expanding due to the growing need for therapies. The market is further expanded by more people seeking therapy due to greater awareness and earlier diagnosis.
Technological developments: The creation of targeted medicines, such as CAR-T cell and gene therapies, provides individualized and possibly effective treatment for a range of blood illnesses, driving market expansion. Drug delivery innovations such as sustained-release formulations and nanomedicines increase patient compliance and treatment efficacy, which increases market potential.
Government Initiatives and Funding: As the prevalence of blood diseases becomes more widely acknowledged, governments across the world are providing funding for research, development, and the availability of reasonably priced medicines. This assistance promotes market expansion by encouraging innovation and facilitating patient access.
Market Restraints
Cost of Treatments: New blood disease medicines, especially gene therapies as well as customized treatments, are frequently expensive. This makes it difficult for individuals and healthcare systems to pay them, which restricts market growth. Limited insurance coverage and reimbursement regulations might further limit a patient's access to costly therapies.
Intellectual Property (IP) Challenges: In the market for blood disease treatments, complicated patent landscapes and possible infringement concerns can impede innovation and provide obstacles for new competitors. This may prevent patients from accessing cutting-edge therapeutic alternatives and impede market expansion. Collaborative research methodologies and cooperative alliances can assist get around IP issues and hasten the creation of novel treatments.
Limited Market Size: Larger pharmaceutical firms find it less appealing to spend on research and development when the patient population is smaller since the potential sales volume for a new medication is reduced. This may hinder creativity and narrow the path to novel treatments. In comparison to major therapeutic regions, smaller markets could not provide the same degree of financing, infrastructure, and resources.
Healthcare Policies and Regulatory Landscape
Singapore's healthcare system is well known for its efficiency, user-friendliness, and emphasis on preventative care. At the core of this system is the Health Sciences Authority (HSA), which is necessary to ensure the efficacy and safety of medical items. As Singapore's regulatory body for pharmaceuticals, medical devices, and health-related products, HSA keeps a careful eye on these goods' quality and compliance with international standards. This adherence to stringent regulations supports the public's confidence in Singapore's healthcare system. The government's proactive approach to healthcare management is characterized by its emphasis on health promotion and sickness prevention. The mix of public and private healthcare providers fosters a complete and patient-centric approach, ensuring that medical treatments are not only available to all citizens in Singapore but also comprehensive. Moreover, the application of technology-driven solutions such as electronic health records and telemedicine enhances the effectiveness and efficiency of healthcare delivery. Singapore's healthcare model, which effectively balances innovation, regulation, and accessibility, is a global model for efficient healthcare systems, with the HSA at its core.
Competitive Landscape
Key Players:
- Takeda
- Sanofi
- Novo Nordisk
- CSL Ltd
- Pfizer
- Bayer
- BioLife Sciences
- Agenus
- Spark Therapeutics
- Abeo Therapeutics
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Singapore Blood Disorder Therapeutics Market Segmentation
By Disorder:
- Anemia
- Hemophilia
- Leukemia
- Myeloma
- Lymphoma
- Rare blood disorders
By Product Type
- Plasma-derived therapeutics
- Recombinant therapeutics
- Gene therapy
- Other therapies
By End User
- Hospitals
- Specialty clinics
- Ambulatory care
- Home healthcare
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.