Singapore Atopic Dermatitis Therapeutics Market Analysis
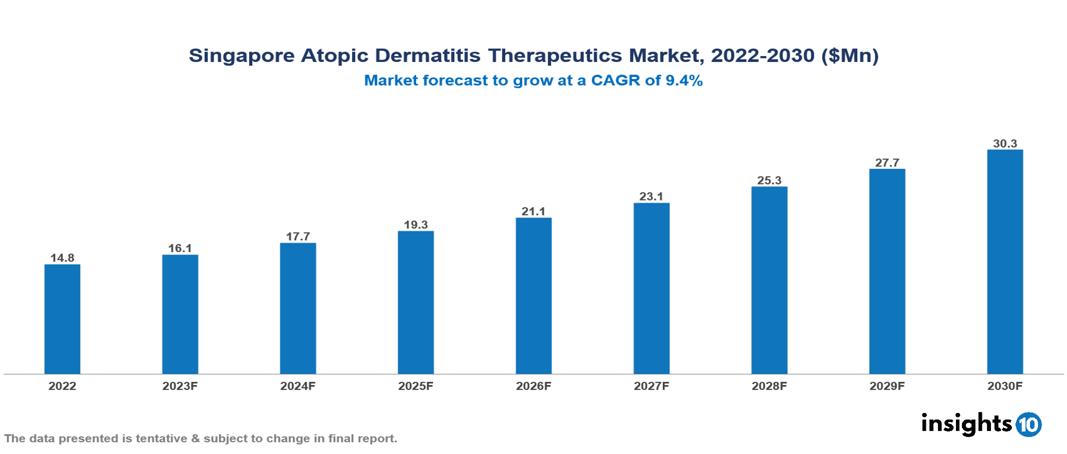
The Singapore Atopic Dermatitis Therapeutics Market was valued at US $15 Mn in 2022, and is predicted to grow at (CAGR) of 9.4% from 2023 to 2030, to US $30 Mn by 2030. The key drivers of this industry include the increased burden of Atopic Dermatitis (AD), strong healthcare infrastructure, supportive government policies, and others. The industry is primarily dominated by players such as Sanofi, Galderma, Merck, AbbVie, Glenmark, Novartis, and Johnson & Johnson, among others.
Buy Now

Singapore Atopic Dermatitis Therapeutics Market Analysis: Executive Summary
The Singapore Atopic Dermatitis Therapeutics Market is at around US $15 Mn in 2022 and is projected to reach US $30 Mn in 2030, exhibiting a CAGR of 9.4% during the forecast period.
Atopic dermatitis, commonly known as eczema, is a persistent skin condition characterized by inflammation, redness, and irritation. It is not contagious and can impact individuals of all age groups, often manifesting in childhood. Key risk factors include a personal or familial history of eczema, allergies, hay fever, or asthma. Common symptoms involve intense itching, redness, swelling, cracking, clear fluid discharge, crusting, and scaling. Treatment approaches for atopic dermatitis may encompass regular moisturizing and self-care practices, the use of medicated creams to alleviate itching and support skin repair, the application of topical or oral medications, and additional therapies like phototherapy or immunosuppressants. Several companies, including Sanofi, Pfizer, AbbVie, Leo Pharma, Eli Lilly, and Novartis, provide therapeutic options for atopic dermatitis, comprising topical corticosteroids, calcineurin inhibitors, and other medications designed to alleviate the symptoms of this skin condition.
The estimated overall prevalence of atopic dermatitis (AD) is around 13.8% in Singapore. The market is being propelled by crucial factors such as the increasing prevalence of AD, strong healthcare infrastructure, and technological advancements in therapeutics industry. However, conditions such as limited affordability, a complex regulatory environment, and adverse environmental conditions limit the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Surge in prevalence of AD: The overall prevalence of AD is estimated to be approximately 13.8% in Singapore. The escalating prevalence of atopic dermatitis, particularly among children, generates an increasing need for efficacious treatment alternatives. This growing burden can be attributed to diverse factors, such as urbanization, hygiene practices, and genetic predisposition.
Strong healthcare infrastructure: Singapore possesses a highly developed healthcare infrastructure featuring advanced medical facilities and a proficient workforce. This facilitates convenient access to specialists, state-of-the-art diagnostic tools, and effective treatment options, collectively contributing to the growth of the market.
Government initiatives: Government initiatives focused on increasing awareness, educating healthcare professionals, and supporting research on atopic dermatitis play a pivotal role in fostering market growth. These programs also enhance diagnosis, set standards for treatment, and improve the accessibility of care.
Advancement in therapeutics: The emergence of novel, safer, and more effective medications, such as targeted biologics and topical formulations, creates fresh opportunities for addressing atopic dermatitis. This innovation attracts investments and broadens the horizons of the market.
Market Restraints
Limited affordability: Sustained management of atopic dermatitis over the long term, involving medications, moisturizers, and emollients, imposes a significant financial burden. Even with public healthcare coverage, elevated out-of-pocket expenses may dissuade patients from seeking regular treatment.
Regulatory hurdles: The stringent regulatory framework in Singapore for the approval of new drugs may postpone their introduction to the market, hindering access to potentially more effective treatments. The prevalence of generic treatments, while enhancing affordability, may also diminish the motivation for the research and development of innovative therapies.
Adverse environmental conditions: The hot and humid climate in Singapore may worsen the symptoms of atopic dermatitis, necessitating additional management strategies and potentially raising treatment costs.
Healthcare Policies and Regulatory Landscape
Singapore's healthcare regulatory landscape is overseen by the Health Sciences Authority (HSA). HSA is the main regulatory body responsible for ensuring the safety, quality, and efficacy of pharmaceuticals, medical devices, and health products in Singapore. Companies looking to introduce new drugs to the Singaporean market must comply with HSA's regulatory processes, which include thorough evaluation and approval procedures. The agency plays a crucial role in the registration and licensing of pharmaceutical products, ensuring they meet stringent standards for safety and effectiveness.
For new entrants in the Singaporean healthcare market, the regulatory environment is generally considered transparent and efficient. Obtaining licenses for pharmaceutical products involves submitting comprehensive documentation to HSA, demonstrating the safety, efficacy, and quality of the products. Singapore's regulatory framework is designed to encourage innovation and investment in the healthcare sector
Competitive Landscape
Key Players
- Novartis
- AbbVie
- Sanofi
- GlaxoSmithKline
- Pfizer
- Johnson & Johnson
- Merck Sharp & Dohme
- Pierre Fabre
- Galderma
- Glenmark
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Singapore Atopic Dermatitis Therapeutics Market Segmentation
By Drug Class
- Corticosteroids
- Calcineurin Inhibitors
- Immunosuppressants
- Biologic Therapy
- PDE-4 Inhibitor
- Antibiotics
- Antihistamines
- Emollients
By Route of Administration
- Topic
- Oral
- Injectable
By Severity type
- Mild
- Moderate
- Severe
By Age Group
- 18 years and below
- 19 years and above
By Distribution channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Dermatology Clinics
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.