Saudi Arabia HIV Drugs Market Analysis
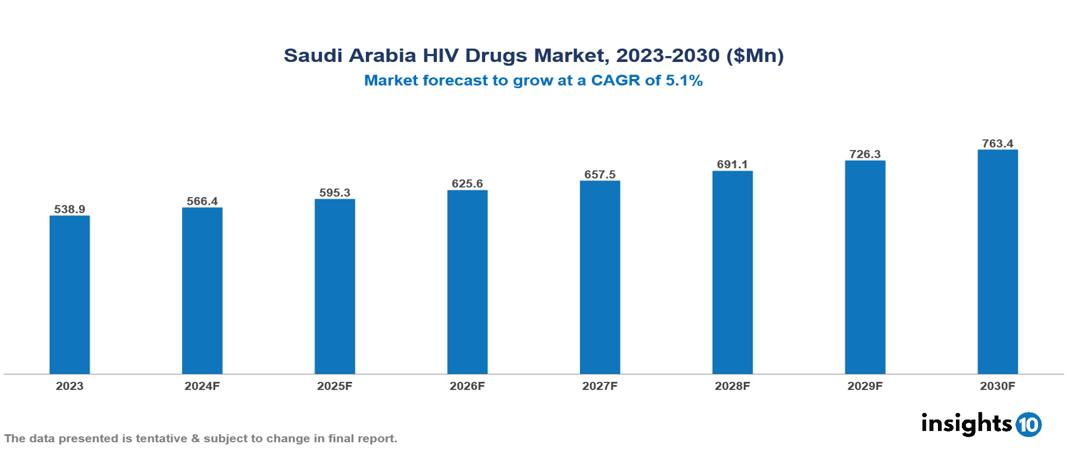
Saudi Arabia HIV Drugs Market is at around $0.54 Bn in 2023 and is projected to reach $0.76 Bn in 2030, exhibiting a CAGR of 5.1% during the forecast period. The market is growing as a result of rising HIV prevalence, government initiatives, and improvements in treatment. The market is dominated by key players like Tabuk Pharmaceuticals, Jamjoom Pharma, ViiV Healthcare, Gilead Sciences, Boehringer Ingelheim International GmbH, Janssen Pharmaceuticals, Merck & Co., Bristol-Myers Squibb, AbbVie Inc., and F. Hoffman-La Roche Ltd.
Buy Now

Saudi Arabia HIV Drugs Market Executive Summary
Saudi Arabia HIV Drugs Market is at around $0.54 Bn in 2023 and is projected to reach $0.76 Bn in 2030, exhibiting a CAGR of 5.1% during the forecast period.
Antiretroviral drugs used to treat HIV/ AIDS are the focus of the Saudi Arabian HIV Drugs Market. This market, which reflects the nation's changing healthcare environment and government efforts to battle HIV/ AIDS, includes the manufacture, distribution, and use of medications intended to manage the illness. The dynamics of this industry in Saudi Arabia are shaped by elements like accessibility, awareness campaigns, and developments in drug development.
Driven by the rising prevalence of HIV/ AIDS, government initiatives, and growing awareness, the HIV medicine industry in Saudi Arabia is expanding rapidly. To offer more advanced therapies, major firms are concentrating on growing their product lines, improving accessibility, and allocating resources to research and development. In the upcoming years, regulatory changes and partnerships with healthcare providers will further accelerate industry expansion.
The global HIV medicine industry has grown rapidly, with a value estimated at $31.7 Bn in 2023. Rising HIV diagnosis rates, as well as government attempts to raise HIV management knowledge, have benefited the HIV medicines market. The market landscape is shifting as generic competition increases. The route forward depends on removing financial barriers, preventing stigma, and expanding access to therapy in developing countries.
Tabuk Pharmaceuticals, Saudi Arabia's largest privately held pharmaceutical company, is a major player in the country's HIV medicine industry. The Saudi HIV medication market is predicted to be valued at $320 Mn and will expand at a CAGR of 5.5% between 2023 and 2028.
Market Dynamics
Market Growth Drivers:
Increasing HIV Prevalence: An increase in HIV prevalence in Saudi Arabia fuels the market's expansion by increasing demand for HIV medications.
Government Initiatives: The demand for HIV medications increases as a result of government initiatives to combat HIV/ AIDS through education campaigns, preventative programs, and enhanced healthcare facilities.
Treatment Advancements: Discoveries in HIV medication therapy and technological developments result in the release of new, more potent medications, which increases market share.
Market Restraints:
Stigma and Discrimination: Despite efforts to minimize stigma and prejudice toward HIV/ AIDS, it persists in many societies, including Saudi Arabia. This stigma may deter people from obtaining testing, treatment, and support services, reducing the market potential for HIV medications.
Cultural and Religious Factors: Cultural and religious beliefs can shape attitudes about HIV/ AIDS and healthcare-seeking behavior. In Saudi Arabia, where cultural norms and religious practices have a considerable influence on public views, these factors may affect HIV medicine acceptance and use.
Restricted Access to Healthcare Services: Despite advancements in healthcare infrastructure, access to healthcare services, particularly HIV testing and treatment, may be limited in some areas of Saudi Arabia. Barriers such as geographic remoteness, financial restraints, and legal restrictions may prohibit people from getting the HIV medications they need.
Healthcare Policies and Regulatory Landscape
An administrative document has been developed by the Saudi Food & medication Authority's (SFDA) Drug Sector to assist stakeholders in the application and authorization procedure for various medication products. When evaluating and submitting medication applications, the SFDA prioritizes quality, safety, and efficacy. After reviewing the medications, the Ministry of Health decides whether to allow their integration into the nation's healthcare system. This is the second stage in Saudi Arabia's drug approval procedure. Businesses may have difficulties since Technical File Preparation requires a significant amount of data collecting that is often of a higher caliber than what is required internationally. Arabic labeling and package translation are also necessary.
Competitive Landscape
Key Players:
- Tabuk Pharmaceuticals
- Jamjoom Pharma
- ViiV Healthcare
- Gilead Sciences
- Boehringer Ingelheim International GmbH
- Janssen Pharmaceuticals
- Merck & Co.
- Bristol-Myers Squibb
- AbbVie Inc.
- F. Hoffman-La Roche Ltd.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
HIV Drugs Market Segmentation
By Drug Class
- Integrase Inhibitors
- Non- Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
- Combination HIV Medicines
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































