Saudi Arabia Congestive Heart Failure Therapeutics Market Analysis
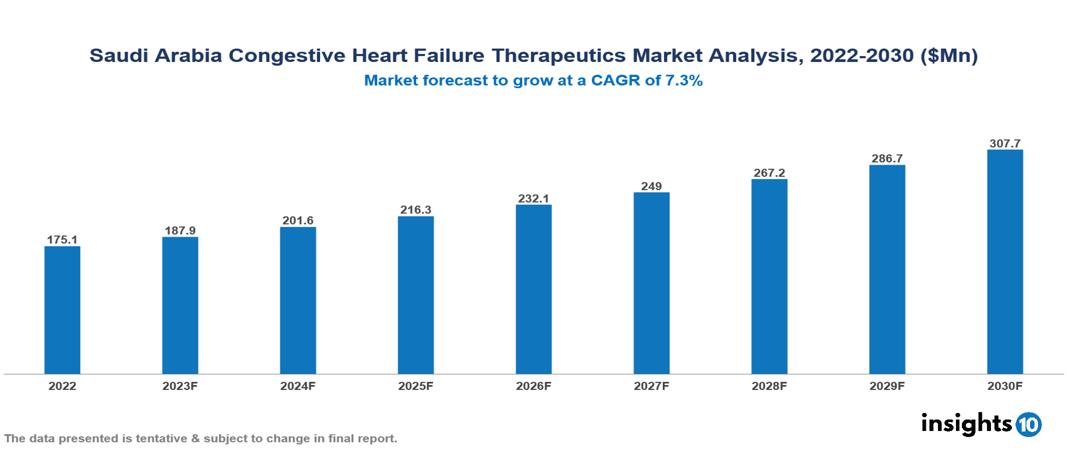
The Saudi Arabia Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $175 Mn in 2022 to $308 Mn by 2030, with a CAGR of 7.3% during the forecast period of 2022-2030. The market is propelled due to the confluence of certain factors like the rapidly increasing prevalence of CHF among the population, the increase in research leading to advances in medical technologies that provide more treatment options, and the increase in public awareness about the risk factors and the disease. The Saudi Arabia Congestive Heart Failure Therapeutics Market has various key players across different therapeutic segments, including Johnson & Johnson, Merck, Novartis, Pfizer, Sanofi, AstraZeneca, Tabuk Pharmaceuticals, Riyadh Pharma, Avalon Pharma, Jamjoom Pharma, etc, among various others.
Buy Now

Saudi Arabia Congestive Heart Failure Therapeutics Market Analysis Executive Summary
The Saudi Arabia Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $175 Mn in 2022 to $308 Mn by 2030, with a CAGR of 7.3% during the forecast period of 2022-2030.
Congestive heart failure (CHF) is a chronic condition in which the heart cannot pump blood effectively, leading to fluid buildup in the lungs and other tissues. The main types are diastolic heart failure, where the ventricles have difficulty relaxing between beats, and systolic heart failure, where the ventricles cannot contract strongly enough to pump out blood. The therapy of CHF frequently involves a combination of pharmaceutical medications, lifestyle changes, and, in extreme cases, surgical operations. Beta-blockers can improve heart function, ACE inhibitors can lower blood pressure, and diuretics can minimize fluid retention. A common focus of lifestyle modifications should be on exercise, weight control, and food adjustments. Technological developments have had a major impact on CHF management. Healthcare practitioners can remotely monitor vital signs and adjust treatment programs with the use of wearable technologies and remote monitoring equipment. Pacemakers and defibrillators are examples of implanted technology that helps control cardiac rhythm and ultimately enhances cardiac function.
The market is propelled due to the confluence of certain factors like the rapidly increasing prevalence of CHF among the population, the increase in research leading to advances in medical technologies that provide more treatment options, and the increase in public awareness about the risk factors and the disease.
In Saudi Arabia, the older population has a higher prevalence of CHF, primarily due to ischemic heart disease and hypertension. The average direct medical cost for a patient with CHF is around $9,500, with hospitalization expenses accounting for the majority of overall spending. These were followed by costs for medication and diagnostics. With the advancement of the illness, the expenses rise dramatically.
Prominent pharmaceutical companies include Novartis (via its partnership with Bristol-Myers Squibb for Entresto), AbbVie, Merck, AstraZeneca, and Pfizer. These companies occupy a larger market share because of their widespread presence and globally known brands. Major local players like Saudi Pharmaceutical Industries and Riyadh Pharma have a smaller market share than global giants because of their limited local presence.
Market Dynamics
Market Growth Drivers
Growing CHF Prevalence: As Saudi Arabia's population is aging quickly, there is a notable rise in the number of older people, who are more vulnerable to CHF. This is brought on by things like longer life expectancies and fewer births. Over 35% of adults in Saudi Arabia are considered fat, which is an extremely high percentage. Being overweight puts a lot of strain on the heart and raises blood pressure, which makes it a major risk factor for CHF.
Increasing Public Awareness about CHF: In Saudi Arabia, public health campaigns and education programs are increasing knowledge of CHF. People are now more equipped to identify symptoms, get an early diagnosis, and comprehend available treatments. Early diagnosis and action are essential for CHF to be adequately managed and complications to be avoided. Raising awareness results in quicker diagnosis, which drives up the number of people starting treatment and expands the market.
Development of New CHF Drugs: The global pharmaceutical industry is actively developing new and innovative drugs for CHF management. These drugs aim to improve patient outcomes, reduce complications, and offer better treatment options. Some of these drugs are in the final stages of their clinical trials and they are expected to be launched in the Saudi market soon. This will provide healthcare providers and patients with more choices and potentially more effective treatments.
Rising Awareness about CHF: Public health initiatives and educational campaigns are raising awareness about CHF in Saudi Arabia. This helps people to recognize symptoms, encourages them to seek early diagnosis, and understand treatment options. Early diagnosis and intervention are crucial for managing CHF effectively and preventing related complications. Increased awareness leads to more timely diagnoses, which translates to higher treatment initiation rates and market growth.
Market Restraints
Exorbitant Cost of Treatment: CHF treatments, particularly novel therapies, can be expensive, putting a heavy financial strain on both patients and the healthcare system in the country. Due to financial constraints, patients may not refill their doses, which reduces the efficacy of their therapy and increases the chances of recurrence. Because of the high expense, people could be discouraged from getting a diagnosis and treatment promptly, which could aggravate their health and cause subsequent, more costly procedures.
Reimbursement Difficulties: In Saudi Arabia, the reimbursement procedure for CHF medications can be convoluted and drawn out, requiring several processes and approvals. Even in cases when a prescription is clinically necessary, doctors may be discouraged from writing it due to the administrative complexity. Patients may be hesitant to seek therapy due to the ambiguity and delays around reimbursement, particularly if they are struggling financially.
Lack of qualified Healthcare workers: In rural and isolated locations in particular, there is a scarcity of qualified healthcare staff trained in the diagnosis and management of CHF. Insufficient proficiency may result in missed diagnosis or inappropriate initial treatment strategies, hence exacerbating patient outcomes. Patients in underprivileged communities might not have access to the drug management and specialist care needed for the management of chronic heart failure.
Healthcare Policies and Regulatory Landscape
The Saudi Food and Drug Authority (SFDA) is an independent body of Saudi Arabia that aims to ensure food and drug safety for the nation. In Saudi Arabia, SFDA plays a critical role in maintaining public health and guaranteeing the efficacy and safety of medications, food, and medical equipment. Its main goal, as an agency of the country’s government, is to supervise and control a number of industries, including food, cosmetics, medical devices, pharmaceuticals, and biologics. In order to guarantee that items entering the Saudi market meet stringent quality and safety requirements and are compliant with international norms, the SFDA oversees the registration, licensing, and approval procedures. The authority plays a critical role in consumer protection through the enforcement of rules, inspections, and ongoing monitoring of healthcare product lifecycles. In addition to its regulatory responsibilities, the SFDA actively supports research and development in the healthcare industry, advancing the local pharmaceutical and medical sectors via innovation and adherence to international best practices.
Notable Recent Updates
June 2023, SFDA approved Amarin’s VASCEPA (icosapent ethyl) capsules for reducing cardiovascular CV risk. It is indicated as an adjunct to statin therapy for use in adult patients with elevated levels of triglycerides TG.
Competitive Landscape
Key Players:
- Johnson & Johnson
- Merck
- Novartis
- Pfizer
- Sanofi
- AstraZeneca
- Tabuk Pharmaceuticals
- Riyadh Pharma
- Avalon Pharma
- Jamjoom Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Saudi Arabia Congestive Heart Failure Therapeutics Market Segmentation
By Stage of Heart Failure
- Acute Heart Failure
- Chronic Heart Failure
By Drug Class
- ACE Inhibitors
- Beta Blockers
- Angiotensin 2 Receptor Blockers
- Diuretics
- Aldosterone Antagonists
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.