Saudi Arabia Central Nervous System (CNS) Therapeutics Market Analysis
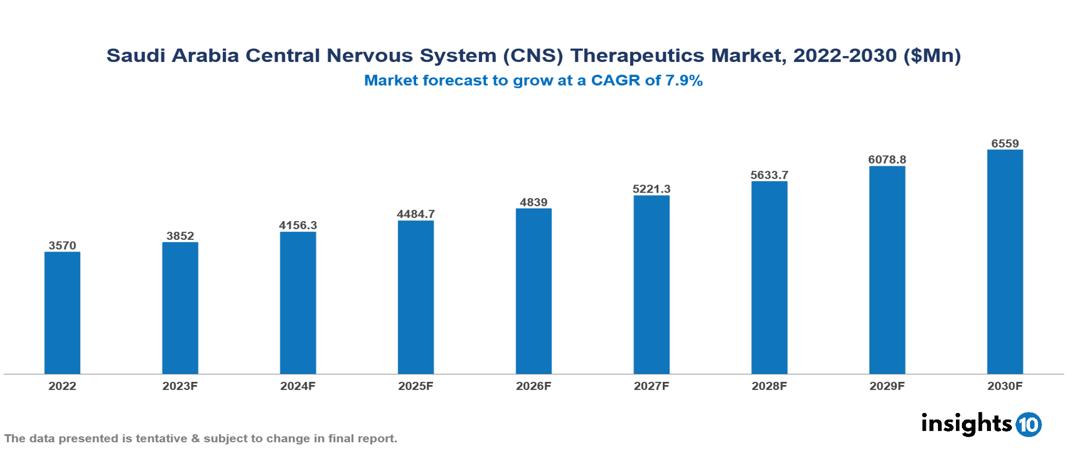
The Saudi Arabia Central Nervous System (CNS)Therapeutics Market was valued at $3.570 Bn in 2022 and is predicted to grow at a CAGR of 7.9% from 2023 to 2030, to $6.559 Bn by 2030. The key drivers of this industry include the rising burden of CNS diseases, expanding healthcare spending, and technological advancements in the industry. The industry is primarily dominated by players such as Pfizer, Novartis, Hikma Pharmaceutical, Otsuka, AbbVie, and Eli Lilly among others.
Buy Now

Saudi Arabia Central Nervous System (CNS) Therapeutics Market Executive Summary
The Saudi Arabia Central Nervous System (CNS)Therapeutics Market is at around $3.570 Bn in 2022 and is projected to reach $6.559 Bn in 2030, exhibiting a CAGR of 7.9% during the forecast period.
Neurological disorders encompass a wide range of conditions affecting both the central and peripheral nervous system, which includes the brain, spinal cord, and nerves. These disorders give rise to a variety of symptoms, such as headaches, limb numbness or weakness, dizziness, cognitive difficulties, speech and vision problems, and tremors. Common instances of neurological disorders include Alzheimer's disease, epilepsy, multiple sclerosis, and Parkinson's disease. The origins of these disorders are diverse, involving factors like genetics, infections, lifestyle-related issues, environmental influences, and underlying health conditions. Treatment approaches for neurological disorders are diverse and may encompass the use of medications, physical therapy, occupational therapy, and, in specific cases, surgical interventions. Companies actively participating in the production, research, and development of solutions for neurological disorders include UCB, Eisai, Biogen, Novartis, and Roche, showcasing a dynamic landscape of initiatives to address these conditions.
The estimated prevalence of neurological disorders ranges from around 3% to 40% for the Saudi population. The market therefore is propelled by major factors like the rising prevalence of CNS disorders, expanding healthcare spending, and technological advancements in the industry. However, conditions such as limited R&D, stringent regulatory environment, lack of awareness, and others limit the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Rising prevalence of CNS diseases: The aging of the Saudi population is elevating the susceptibility to age-related neurological disorders such as Alzheimer's disease, Parkinson's disease, and stroke. The prevalence of neurological disorders is about 3% in Saudi children whereas mental health disorders affect around 40% of youth. The rapid progression of urbanization generates heightened risk factors like obesity, sedentary lifestyles, and unhealthy food habits, which correlate with a growing incidence of mental health problems, such as depression and anxiety.
Expanding healthcare spending: The Saudi government's Vision 2030 project has a primary emphasis on expanding healthcare, which includes increased funding for neurological research, improved access to pharmaceuticals, and setting up specialist treatment centers. Government-sponsored public health awareness campaigns are actively encouraging individuals to seek assistance for neurological disorders and mental health, thereby fostering a growing demand for treatment.
Technological advancements: Current research is concentrated on the development of novel, enhanced, and individualized treatments for CNS disorders. The possibility of gene therapy and precision medicine allows tailored and potentially curative therapies for a variety of CNS problems.
Market Restraints
Lack of human resources: A shortage of proficient mental health professionals restricts the ability to accurately diagnose and treat CNS disorders. Inadequate education in CNS therapeutics contributes to healthcare professionals lacking the specialized knowledge and skills necessary for the proper administration and monitoring of advanced CNS medications.
Lack of awareness: A considerable number of individuals suffering from CNS disorders go undetected as a result of insufficient awareness about symptoms and a reluctance to seek assistance. The stigma associated with mental health exacerbates this issue, dissuading individuals from pursuing necessary treatment.
Limited R&D: Insufficient funding allocated to local research and development in CNS therapeutics hinders the creation of novel and innovative treatment alternatives designed to address the unique requirements of the Saudi population.
Stringent regulatory environment: The registration procedure for novel CNS medications can be protracted and intricate, impeding the availability of innovative treatment choices. Stringent pricing controls, such as government-imposed regulations, may restrict the profitability of CNS medications, potentially dissuading pharmaceutical companies from venturing into the market.
Healthcare Policies and Regulatory Landscape
The regulatory authority for therapeutics in Saudi Arabia is the Saudi Food and Drug Authority (SFDA). SFDA is responsible for regulating and overseeing the safety, efficacy, and quality of pharmaceuticals and medical devices in the Kingdom. It plays a crucial role in ensuring that therapeutic products meet the necessary standards and comply with regulatory requirements before they are introduced into the Saudi market.
The process of obtaining licensure for therapeutics in Saudi Arabia typically involves a thorough evaluation by SFDA. If the therapeutic product meets the necessary criteria, SFDA grants marketing authorization, allowing the product to be legally distributed and sold in Saudi Arabia.
For new entrants into the therapeutic market in Saudi Arabia, navigating the regulatory landscape may require careful consideration of SFDA's requirements. While the regulatory environment aims to safeguard public health, it also demands diligence and adherence to established procedures, which may pose challenges for new entrants.
Competitive Landscape
Key Players
- Pfizer Inc.
- AbbVie
- Biogen
- Novartis AG
- Eli Lilly
- Merck & Co
- AstraZeneca
- Hikma Pharmaceutical
- Otsuka
- Saudi Pharmaceutical Industries
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Saudi Arabia Central Nervous System (CNS)Therapeutics Market Segmentation
By Drug
- Biologics
- Non-Biologics
By Drug Class
- Antidepressants
- Analgesics
- Immunomodulators
- Interferons
- Decarboxylase Inhibitors
- Others
By Disease
- Neurovascular Disease
- Degenerative Disease
- Infectious Disease
- Mental Health
- CNS Cancer
- Others
By Distribution Channel
- Hospital-based pharmacies
- Retail pharmacies
- Online pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.